Age-related mesenchymal stromal cell senescence is associated with progression from MGUS to multiple myeloma
- PMID: 40263435
- PMCID: PMC12133572
- DOI: 10.1038/s41375-025-02621-7
Age-related mesenchymal stromal cell senescence is associated with progression from MGUS to multiple myeloma
Abstract
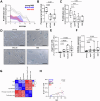
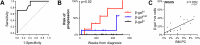
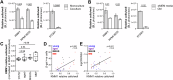
The risk of progression of monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma (MM) increases with advancing age, suggesting that progression may be influenced by age-related changes within the bone marrow (BM) microenvironment. We hypothesise that senescent mesenchymal stromal cells (MSCs), which accumulate in the BM with age, may contribute to MGUS progression to MM. Here, we show that, like BM MSCs from aged non-cancer controls, BM MSCs from both MM and MGUS patients exhibit a senescent phenotype characterised by enlarged, flattened morphology, increased β-galactosidase activity and CDKN2A expression, and decreased proliferation rate compared with BM MSCs from healthy young individuals. While coculture with BM MSCs suppresses the proliferative capacity of MM cell lines in vitro, induction of senescence via irradiation or replicative exhaustion in healthy MSCs relieves this suppression, compared with non-senescent MSCs. This may, in part, be attributable to upregulated expression of the BMP antagonist Gremlin1 in senescent MSCs, which facillitates MM cell proliferation. Notably, the risk of progression to MM was significantly elevated in MGUS patients with increased MSC senescence. Collectively, our data provide evidence that age-related accumulation of senescent MSCs may be a driver of MGUS to MM progression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691–97. - PubMed
-
- Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E, et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol. 2002;20:1625–34. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

