Antisense oligonucleotide treatment in a preterm infant with early-onset SCN2A developmental and epileptic encephalopathy
- PMID: 40263630
- PMCID: PMC12283366
- DOI: 10.1038/s41591-025-03656-0
Antisense oligonucleotide treatment in a preterm infant with early-onset SCN2A developmental and epileptic encephalopathy
Abstract
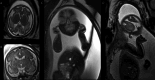
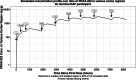
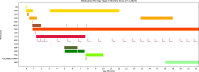
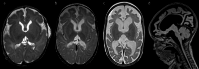
Early-onset SCN2A developmental and epileptic encephalopathy is caused by SCN2A gain-of-function variants. Here we describe the clinical experience with intrathecally administered elsunersen, a gapmer antisense oligonucleotide targeting SCN2A, in a female preterm infant with early-onset SCN2A developmental and epileptic encephalopathy, in an expanded access program. Before elsunersen treatement, the patient was in status epilepticus for 7 weeks with a seizure frequency of 20-25 per hour. Voltage-clamp experiments confirmed impaired channel inactivation and increased persistent current consistent with a gain-of-function mechanism. Elsunersen treatment demonstrated a favorable safety profile with no severe or serious adverse events reported after 19 intrathecal administrations over 20 months. After administration in combination with sodium channel blockers, status epilepticus was interrupted intermittently and ultimately ceased after continued dosing. A >60% reduction in seizure frequency corresponding to five to seven seizures per hour was observed, which has been sustained during follow-up until the age of 22 months. These data provide preliminary insights on the safety and efficacy of elsunersen in a preterm infant. Additional investigation on the benefits of elsunersen in clinical trials is warranted.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Elsunersen was provided by Praxis Precision Medicines within an expanded access program with authors being employees of Praxis Precision Medicines (S.F., R.H., H.J., W.M., B.S., S.P. and M.So.). The other authors declare no competing interests.
Figures




References
-
- Wolff, M. et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain140, 1316–1336 (2017). - PubMed
-
- Hedrich, U. B. S., Lauxmann, S. & Lerche, H. SCN2A channelopathies: mechanisms and models. Epilepsia60, S68–S76 (2019). - PubMed
-
- Berkovic, S. F. et al. Benign familial neonatal–infantile seizures: characterization of a new sodium channelopathy. Ann. Neurol.55, 550–557 (2004). - PubMed
-
- Liao, Y. et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology75, 1454–1458 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

