Woodward and Hoffmann. Hoffmann and Woodward. A Close Collaboration Had Yet to Begin
- PMID: 40263921
- PMCID: PMC12067179
- DOI: 10.1002/tcr.202400204
Woodward and Hoffmann. Hoffmann and Woodward. A Close Collaboration Had Yet to Begin
Abstract
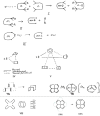
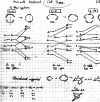
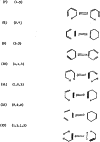
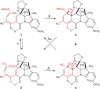
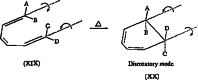
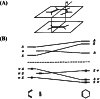
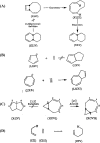
In 1965, R. B. Woodward and Roald Hoffmann published five communications in the Journal of the American Chemical Society in which they outlined the mechanisms of electrocyclizations, cycloadditions, and sigmatropic reactions - today known as the Woodward-Hoffmann rules. Over the next several years, the organic chemistry community rushed to test the validity of the W-H rules and expand the range of reactions covered by them. Meanwhile, Woodward and Hoffmann were besieged with invitations to lecture and write expositions on these concepts. In this publication, I present an analysis of Woodward and Hoffmann's next publications in 1966 and 1967 on the W-H rules. Two of these publications were based on lectures Woodward or Hoffmann presented in late 1965 and 1966. I also discuss their own continuing research on the topic in this time period (all by Hoffmann; none by Woodward). I conclude that the assumed intimate collaboration of Woodward and Hoffmann had actually not yet begun.
Keywords: History of chemistry; Woodward-Hoffmann rules; collaborations; pericyclic reactions.
© 2025 The Author(s). The Chemical Record published by The Chemical Society of Japan and Wiley-VCH GmbH.
Figures









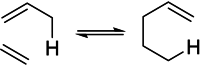





References
-
- R. Hoffmann, video interview with J. I. Seeman, Ithaca, NY, November 14, 2023.
-
- R. Hoffmann, review of manuscript draft and email to J. I. Seeman, Ithaca, NY, September 30, 2024.
-
- Woodward R. B., Hoffmann R., J. Am. Chem. Soc. 1965, 87, 395–397, DOI: 10.1021/ja01080a054. - DOI
-
- Hoffmann R., Woodward R. B., J. Am. Chem. Soc. 1965, 87, 2046–2048, doi.org/10.1021/ja01087a034. - DOI
-
- Woodward R. B., Hoffmann R., J. Am. Chem. Soc. 1965, 87, 2511–2513, doi.org/10.1021/ja01089a050. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

