Pyrotinib and Nab-Paclitaxel in HER2-Positive Breast Cancer (PANHER Trial): A Prospective, Single-Arm, Phase II Trial
- PMID: 40263948
- PMCID: PMC12210037
- DOI: 10.1111/cas.70086
Pyrotinib and Nab-Paclitaxel in HER2-Positive Breast Cancer (PANHER Trial): A Prospective, Single-Arm, Phase II Trial
Abstract
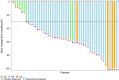
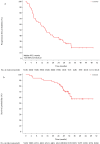
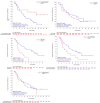
Trastuzumab and pertuzumab combined with chemotherapy represent the standard therapy for first-line treatment of HER2-positive metastatic breast cancer (BC). Due to challenges related to availability and cost in China, it is necessary to explore treatments involving tyrosine kinase inhibitors (TKIs). In this multicenter, single-arm, open-label phase II trial, patients with HER2-positive BC were enrolled from seven hospitals in China. Patients received oral pyrotinib 400 mg once daily and intravenous nab-paclitaxel 125 mg/m2 on days 1, 8, and 15 of each 28-day cycle until disease progression or intolerable toxicity. The primary endpoint was the objective response rate (ORR). Between December 2019 and December 2021, 51 patients were enrolled. Among all enrolled patients, 48 had at least one response evaluation. Of these evaluable patients, 39 patients achieved responses, resulting in a positive study outcome. The ORR was 76.5% (95% CI, 62.5%-87.2%), and the disease control rate was 94.1% (95% CI, 83.8-98.8). As of January 24, 2024, the median follow-up duration was 29.2 months (IQR, 24.9-33.2). The median progression-free survival was 14.6 months (95% CI, 8.0-24.2), and the median overall survival was not reached. Forty-nine patients (96.1%) developed grade ≥ 3 adverse events (AEs). The most common grade ≥ 3 AEs were decreased neutrophil count (43.1%), decreased white blood cell count (43.1%), and diarrhea (23.5%). Pyrotinib combined with nab-paclitaxel demonstrated promising efficacy for HER2-positive advanced breast cancer, with an acceptable safety profile. Trial Registration: chictr.org, ChiCTR1900023653.
Keywords: HER2; breast cancer; nab‐paclitaxel; pyrotinib; tyrosine kinase inhibitor.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Pai Peng is an employee in Jiangsu Hengrui Pharmaceuticals Co. Ltd.
Figures
References
-
- Marty M., Cognetti F., Maraninchi D., et al., “Randomized Phase II Trial of the Efficacy and Safety of Trastuzumab Combined With Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2‐Positive Metastatic Breast Cancer Administered as First‐Line Treatment: The M77001 Study Group,” Journal of Clinical Oncology 23, no. 19 (2005): 4265–4274, 10.1200/jco.2005.04.173. - DOI - PubMed
-
- Swain S. M., Miles D., Kim S. B., et al., “Pertuzumab, Trastuzumab, and Docetaxel for HER2‐Positive Metastatic Breast Cancer (CLEOPATRA): End‐Of‐Study Results From a Double‐Blind, Randomised, Placebo‐Controlled, Phase 3 Study,” Lancet Oncology 21, no. 4 (2020): 519–530, 10.1016/s1470-2045(19)30863-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous