Optimal duration of anticoagulation after left atrial appendage closure: a systematic review and meta-analysis
- PMID: 40264015
- PMCID: PMC12016058
- DOI: 10.1186/s12872-025-04736-2
Optimal duration of anticoagulation after left atrial appendage closure: a systematic review and meta-analysis
Abstract
Background: Left atrial appendage closure (LAAC) has become the treatment of choice for stroke prevention in patients with nonvalvular atrial fibrillation who are at high risk of bleeding or with contraindications for anticoagulation. However, the optimal duration of anticoagulation after LAAC remains uncertain. The aim of this study was to evaluate the optimal duration of treatment with novel oral anticoagulants (NOACs) after LAAC.
Method: We searched the PubMed, Embase, Cochrane Library, and Web of Science databases for studies related to LAAC published from inception to 20 December 2023, and performed a meta-analysis comparing the efficacy and safety of 45-day and 3-month postoperative NOAC treatment using R4.3.1 software.
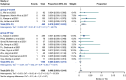
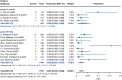
Results: A total of 14 studies were included in this study, of which 4 were prospective cohort studies and 10 were retrospective cohort studies. The incidence of stroke or transient ischaemic attack (0.018 [95% CI: 0.007-0.033] in the 3-month group and 0.005 [95% CI: 0.001-0.011] in the 45-day group; P = 0.07) and the incidence of device-related thrombus (0.025 [95% CI: 0.002-0.065] in the 3-month group and 0.020 [ 95% CI: 0.007-0.037] in the 45-day group; P = 0.81) were not significantly different. However, the incidence of major bleeding was significantly greater in the 3-month group than in the 45-day group (0.033 [95% CI: 0.018-0.053] in the 3-month group and 0.003 [95% CI: 0.000-0.008] in the 45-day group; P < 0.01).
Conclusions: Compared with the 3-month scheme, 45 days of postoperative anticoagulation significantly reduced the risk of major bleeding in patients without compromising the efficacy of preventing stroke or transient ischaemic attack and device-related thrombus.
Trial registration: Our meta-analysis was registered in the PROSPERO international database (CRD42024524661).
Keywords: Left atrial appendage closure; Major bleeding; Novel oral anticoagulants; Stroke.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical and IRB approval of this analysis were not required as no human or animal subjects were involved. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Propensity-Matched Comparison of Oral Anticoagulation Versus Antiplatelet Therapy After Left Atrial Appendage Closure With WATCHMAN.JACC Cardiovasc Interv. 2019 Jun 10;12(11):1055-1063. doi: 10.1016/j.jcin.2019.04.004. JACC Cardiovasc Interv. 2019. PMID: 31171282
-
Left atrial appendage closure: A therapy uniquely suited for specific populations of patients with atrial fibrillation.J Cardiovasc Electrophysiol. 2019 Dec;30(12):2968-2976. doi: 10.1111/jce.14182. Epub 2019 Oct 17. J Cardiovasc Electrophysiol. 2019. PMID: 31520437
-
Left atrial appendage closure in patients with atrial fibrillation and acute ischaemic stroke despite anticoagulation.Stroke Vasc Neurol. 2025 Feb 25;10(1):120-128. doi: 10.1136/svn-2024-003143. Stroke Vasc Neurol. 2025. PMID: 38862182 Free PMC article.
-
Comparative Efficacy and Safety of Low-Dose Direct Oral Anticoagulants Versus Dual Antiplatelet Therapy Following Left Atrial Appendage Occlusion in Patients With Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis.Catheter Cardiovasc Interv. 2025 May;105(6):1311-1319. doi: 10.1002/ccd.31461. Epub 2025 Feb 20. Catheter Cardiovasc Interv. 2025. PMID: 39980323
-
Systematic review on left atrial appendage closure with the LAmbre device in patients with non-valvular atrial fibrillation.BMC Cardiovasc Disord. 2020 Feb 12;20(1):78. doi: 10.1186/s12872-020-01349-9. BMC Cardiovasc Disord. 2020. PMID: 32050904 Free PMC article.
Cited by
-
Managing Stroke Risk in Moyamoya Disease With Paroxysmal Atrial Fibrillation: A Case Report.Cureus. 2025 Jun 10;17(6):e85721. doi: 10.7759/cureus.85721. eCollection 2025 Jun. Cureus. 2025. PMID: 40642733 Free PMC article.
References
-
- Holmes DR Jr, et al. “Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial,” (in eng). J Am Coll Cardiol. 2014;64(1):1–12. - PubMed
-
- Reddy VY, et al. “Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial,” (in eng). JAMA. 2014;312(19):1988–98. - PubMed
-
- Reddy VY, et al. “5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials,” (in eng). J Am Coll Cardiol. 2017;70(24):2964–75. - PubMed
-
- Holmes DR, et al. “Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial,” (in eng). Lancet. 2009;374(9689):534–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

