Efficacy of lactoferrin in preventing recurrent urinary tract infections in pregnant Egyptian women: a randomized controlled trial
- PMID: 40264102
- PMCID: PMC12016170
- DOI: 10.1186/s12894-025-01725-7
Efficacy of lactoferrin in preventing recurrent urinary tract infections in pregnant Egyptian women: a randomized controlled trial
Retraction in
-
Retraction Note: Efficacy of lactoferrin in preventing recurrent urinary tract infections in pregnant Egyptian women: a randomized controlled trial.BMC Urol. 2025 Sep 15;25(1):230. doi: 10.1186/s12894-025-01929-x. BMC Urol. 2025. PMID: 40954463 Free PMC article. No abstract available.
Abstract
Background: Prevention of a very prevalent problem such as urinary tract infections (UTIs), is of utmost importance, particularly during pregnancy, in order to limit the irrational use of antibiotics. Lactoferrin (Lf) has proven in vivo and in vitro antibacterial actions, especially against Escherichia coli, which is the main causative organism of UTIs. The study question is, "Does the administration of Lf to pregnant women with a history of RUTIs reduce the incidence of new episodes of UTIs during pregnancy?".
Methods: This was a randomized controlled study over 6 months that started from February 2024 to August 2024, conducted at the antenatal clinic of El-Shatby University Hospital, Alexandria, Egypt. The study included 220 pregnant women (14-24 weeks' gestation) who had experienced two or more UTI episodes in the previous six months. A negative urine culture right before enrollment was an inclusion criterion. Participants were randomly allocated into two groups; 110 women received a daily dose of 200 mg of lactoferrin, and 110 women as controls. Women were followed up by urine cultures and sensitivity monthly, and they were asked to report any symptoms of UTIs present. The outcomes were the number of episodes of asymptomatic bacteriuria (ASB), acute cystitis, or pyelonephritis in both groups.
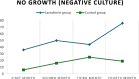
Results: A total of 874 urine samples were collected from women in both groups (438 from the Lf group and 436 from the control group), and their results were analyzed. Over the follow-up period we diagnosed 164 episodes of ASB (33 episodes in the lactoferrin group versus 131 episodes in the control group) and 46 episodes of acute cystitis (4 episodes in the lactoferrin group versus 42 episodes in the control group) were diagnosed. These results were statistically significant (P < 0.00001). No episodes of pyelonephritis were observed in our study cohort. Escherichia coli (E. coli) was the most prevalent isolated organism, accounting for 27% of the ASB episodes in the lactoferrin group and 51% of the ASB episodes in the control group. Similarly, E. coli was isolated in 25% of acute cystitis episodes in the Lf group and 45.2% in the control group. In the exposed group, Lf reduced the risk of both ASB and acute cystitis by 75% and 90%, respectively.
Conclusion: Findings of this study suggest that Lf may play an important preventive role against asymptomatic bacteriuria and symptomatic urinary tract infections in pregnant women. Further multicenter studies on a larger number of patients are needed to improve the generalizability of the results.
Keywords: Lactoferrin; Pregnancy; Prevention; Urinary tract infections.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was ethically approved from the ethical committee of the Faculty of Medicine, Alexandria University (IRB No: 00012098, Approval No: 0107712, on 14th May 2023). The study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant before enrolling in this study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Mohamed NR, Omar HH, Abd-Allah IM. Prevalence and risk factors of urinary tract infection among pregnant women in Ismailia City. Egypt IOSR J Nurs Health Sci. 2017;6(3):62–72. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases