Glucose- and glutamine-driven de novo nucleotide synthesis facilitates WSSV replication in shrimp
- PMID: 40264189
- PMCID: PMC12012963
- DOI: 10.1186/s12964-025-02186-z
Glucose- and glutamine-driven de novo nucleotide synthesis facilitates WSSV replication in shrimp
Abstract
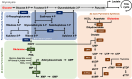
Background: Viruses rely on host metabolism to complete their replication cycle. White spot syndrome virus (WSSV), a major pathogen in shrimp aquaculture, hijacks host metabolic pathways to fulfill its biosynthetic and energetic needs. Previous studies have demonstrated that WSSV promotes aerobic glycolysis (Warburg effect) and glutaminolysis during its replication stage (12 hpi). Therefore, glucose and glutamine serve as crucial metabolites for viral replication. Additionally, de novo nucleotide synthesis, including the pentose phosphate pathway and purine/pyrimidine synthesis, is significantly activated during WSSV infection. However, the precise association between WSSV and host glucose and glutamine metabolism in driving de novo nucleotide synthesis remains unclear. This study aimed to investigate the involvement of glucose and glutamine in nucleotide metabolism during WSSV replication and to elucidate how WSSV reprograms these pathways to facilitate its pathogenesis.
Methods: To assess changes in metabolic flux during WSSV replication, LC-ESI-MS-based isotopically labeled glucose ([U-13C] glucose) and glutamine ([A-15N] glutamine) were used as metabolic tracers in in vivo experiments with white shrimp (Litopenaeus vannamei). The in vivo experiments were also conducted to measure the expression and enzymatic activity of genes involved in nucleotide metabolism. Additionally, in vivo dsRNA-mediated gene silencing was employed to evaluate the roles of these genes in WSSV replication. Pharmacological inhibitors targeting the Ras-PI3K-Akt-mTOR pathway were also applied to investigate its regulatory role in WSSV-induced nucleotide metabolic reprogramming.
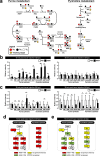
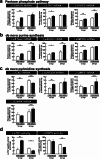
Results: The metabolite tracking analysis confirmed that de novo nucleotide synthesis was significantly activated at the WSSV replication stage (12 hpi). Glucose metabolism is preferentially reprogrammed to support purine synthesis, while glutamine uptake is significantly increased and contributes to both purine and pyrimidine synthesis. Consistently, gene expression and enzymatic activity analyses, along with gene silencing experiments, indicated the critical role of de novo nucleotide synthesis in supporting viral replication. However, while the inhibition of the Ras-PI3K-Akt-mTOR pathway suggested its involvement in regulating nucleotide metabolism, no consistent effect on WSSV replication was observed, suggesting the presence of alternative regulatory mechanisms.
Conclusion: This study demonstrates that WSSV infection induces specific metabolic reprogramming of glucose and glutamine utilization to facilitate de novo nucleotide synthesis in shrimp. These metabolic changes provide the necessary precursors for nucleotide synthesis, supporting WSSV replication and pathogenesis. The findings offer novel insights into the metabolic strategies employed by WSSV and suggest potential targets for controlling WSSV outbreaks in shrimp aquaculture.
Keywords: Pentose phosphate pathway; Warburg effect; White shrimp; White spot syndrome virus; de novo nucleotide metabolism; in vivo stable-isotope tracing metabolomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No specific permits were required for the use of experimental shrimp, as they are invertebrates. All handling and treatment procedures strictly adhered to the institutional ethical guidelines. Consent for publication: All authors are consent for publication. Competing interests: The authors declare no competing interests.
Figures




References
-
- Food and Agriculture Organization of the United Nations. [https://www.fao.org/home/en]. The State of World Fisheries and Aquaculture 2024: Blue Transformation in Action. Rome: FAO; 2024. Available from: https://enaca.org/?id=1337
-
- Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell M. A 20-year retrospective review of global aquaculture. Nature. 2021;591(7851):551–63. 10.1038/s41586-021-03308-6. - PubMed
MeSH terms
Substances
Grants and funding
- NSTC 111-2313-B-006-007-MY3/National Science and Technology Council
- NSTC 111-2313-B-006-007-MY3/National Science and Technology Council
- NSTC 111-2313-B-006-007-MY3/National Science and Technology Council
- NSTC 111-2313-B-006-007-MY3/National Science and Technology Council
- NSTC 111-2313-B-006-007-MY3/National Science and Technology Council
LinkOut - more resources
Full Text Sources
Miscellaneous

