Sildenafil promotes osteogenic differentiation of human mesenchymal stem cells and inhibits bone loss by affecting the TGF-β signaling pathway
- PMID: 40264229
- PMCID: PMC12016470
- DOI: 10.1186/s13287-025-04320-7
Sildenafil promotes osteogenic differentiation of human mesenchymal stem cells and inhibits bone loss by affecting the TGF-β signaling pathway
Abstract
Background: Osteoporosis, a common bone disorder, is primarily managed pharmacologically. However, existing medications are associated with non-trivial side-effects. Sildenafil, which already finds many clinical applications, promotes angiogenesis and cellular differentiation. Osteoporotic patients often exhibit a reduced intraosseous vasculature and impaired cellular differentiation; sildenafil may thus usefully treat osteoporosis.
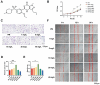
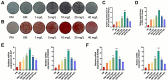
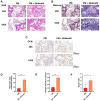
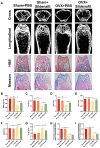
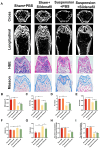
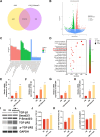
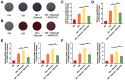
Methods: Here, the effects of sildenafil on the osteogenic differentiation of human mesenchymal stem cells (hMSCs) were explored, as were the molecular mechanisms in play. We treated hMSCs with varying concentrations of sildenafil and measured cell proliferation and osteogenic differentiation in vitro. We used a mouse model of subcutaneous ectopic osteogenesis to assess sildenafil's effect on hMSC osteogenic differentiation in vivo. We also explored the effects of sildenafil on bone loss in tail-suspended (TS) and ovariectomized (OVX) mice. Mechanistically, we employed RNA-sequencing to define potentially relevant molecular pathways.
Results: The appropriate concentrations of sildenafil significantly enhanced osteogenic hMSC differentiation; the optimal sildenafil concentration may be 10 mg/L. Sildenafil mitigated osteoporosis in OVX and TS mice. The appropriate concentrations of sildenafil probably promoted hMSC osteogenic differentiation by acting on the transforming growth factor-β (TGF-β) signaling pathway.
Conclusions: In conclusion, sildenafil enhanced hMSC osteogenic differentiation and inhibited bone loss. Sildenafil may usefully treat osteoporosis. Our findings offer new insights into the physiological effects of the medicine.
Keywords: Mesenchymal stem cells; Osteogenesis; Osteoporosis; Sildenafil; TGF-β signaling pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study titled “The Effects and Mechanisms of Sildenafil in the Treatment of Osteoporosis” was approved by the Laboratory Animal Welfare and Ethics Committee of the Biomedical Ethics Committee at Peking University (Date: 16. 02. 2023, No. LA2023198). The specific animal experimental protocol for this study was pre-designed prior to the research. All surgeries were performed under anesthesia, and all efforts were made to minimize animal suffering. hBMSCs and hASCs were obtained from ScienCell Company (USA). ScienCell Company has confirmed that there was initial ethical approval for collection of human cells, and that the donors had signed informed consent. Consent for publication: Not applicable. Competing interests: The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- 82170929/the National Science Foundation of China
- 82370924/the National Science Foundation of China
- L222030, L222090/the Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund Project
- L222145/the Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund Project
- PKUSS20230101/the Youth Research Fund of Peking University School and Hospital of Stomatology
LinkOut - more resources
Full Text Sources
Medical

