Mechanical cues rewire lipid metabolism and support chemoresistance in epithelial ovarian cancer cell lines OVCAR3 and SKOV3
- PMID: 40264231
- PMCID: PMC12016438
- DOI: 10.1186/s12964-025-02144-9
Mechanical cues rewire lipid metabolism and support chemoresistance in epithelial ovarian cancer cell lines OVCAR3 and SKOV3
Abstract
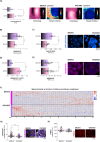
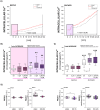
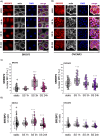
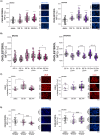
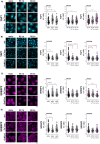
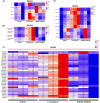
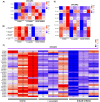
Epithelial ovarian cancer (EOC) is one of the deadliest cancers in women, and acquired chemoresistance is a major contributor of aggressive phenotypes. Overcoming treatment failure and disease recurrence is therefore an ambitious goal. Ovarian cancer develops in a biophysically challenging environment where the cells are constantly exposed to mechanical deformation originating in the abdomen and shear stress caused by the accumulation of ascitic fluid in the peritoneal cavity. Therefore, mechanical stimulation can be seen as an inseparable part of the tumor microenvironment. The role of biomechanics in shaping tumor metabolism is emerging and promises to be a real game changer in the field of cancer biology. Focusing on two different epithelial ovarian cancer cell lines (SKOV3 and OVCAR3), we explored the impact of shear stress on cellular behavior driven by mechanosensitive transcription factors (TFs). Here, we report data linking physical triggers to the alteration of lipid metabolism, ultimately supporting increased chemoresistance. Mechanistically, shear stress induced adaptation of cell membrane and actin cytoskeleton which were accompanied by the regulation of nuclear translocation of SREBP2 and YAP1. This was associated with increased cholesterol uptake/biosynthesis and decreased sensitivity to the ruthenium-based anticancer drug BOLD-100. Overall, the present study contributes to shedding light on the molecular pathways connecting mechanical cues, tumor metabolism and drug responsiveness.
Keywords: BOLD-100; Lipid metabolism; Mechanosensitive transcription factors; Mechanotransduction; Ovarian cancer; SREBP2; YAP1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Yes. Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

