Competition between elasticity and adhesion in caterpillar locomotion
- PMID: 40264359
- PMCID: PMC12015574
- DOI: 10.1098/rsif.2024.0703
Competition between elasticity and adhesion in caterpillar locomotion
Abstract
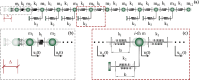
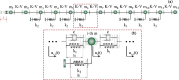
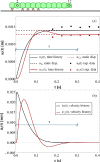
In recent years, there has been a growing interest in understanding animals' locomotion mechanisms for developing bio-inspired micro- or nano-robots capable of overcoming obstacles and navigating in confined environments. Among non-pedal crawlers, caterpillars exhibit one of the most stable and efficient gait strategies, utilizing muscle contractions and substrate grip. Although several approaches have been proposed to model their locomotion, little is known about the competition between body elasticity and adhesion, which we demonstrate playing a central role in crawling gait. Preliminarily, experimental observations and measurements were performed on Pieris brassicae larvae, gaining insights into fundamental features characterizing caterpillar locomotion and estimating key geometrical and mechanical parameters. A minimal but effective one-dimensional discrete model was thus conceived to capture all the relevant aspects of the movement. Inter-mass springs model the deformable body units, Winkler-like constraints with an adhesion threshold reproduce elastic interactions and attaching/detaching events at prolegs-substrate interface, and a triggering muscle contraction initiates the larva's crawling cycle, generating the observed travelling wave. After demonstrating theoretically that caterpillars move obeying quasi-static laws, we proved robustness of the proposed approach by showing very good agreement between theoretical outcomes and experimental evidence, so paving the way for new optimization strategies in soft robotics.
Keywords: caterpillar locomotion; crawling gait mechanics; interplay elasticity–adhesion.
Conflict of interest statement
We declare we have no competing interests.
Figures










Similar articles
-
Bone-free: soft mechanics for adaptive locomotion.Integr Comp Biol. 2014 Dec;54(6):1122-35. doi: 10.1093/icb/icu076. Epub 2014 Jun 18. Integr Comp Biol. 2014. PMID: 24944114
-
Modeling locomotion of a soft-bodied arthropod using inverse dynamics.Bioinspir Biomim. 2011 Mar;6(1):016001. doi: 10.1088/1748-3182/6/1/016001. Epub 2010 Dec 15. Bioinspir Biomim. 2011. PMID: 21160115
-
Locomotion in caterpillars.Biol Rev Camb Philos Soc. 2014 Aug;89(3):656-70. doi: 10.1111/brv.12073. Epub 2014 Jan 10. Biol Rev Camb Philos Soc. 2014. PMID: 24405585 Review.
-
Modeling of caterpillar crawl using novel tensegrity structures.Bioinspir Biomim. 2012 Dec;7(4):046006. doi: 10.1088/1748-3182/7/4/046006. Epub 2012 Aug 8. Bioinspir Biomim. 2012. PMID: 22872665
-
Flexible mechanisms: the diverse roles of biological springs in vertebrate movement.J Exp Biol. 2011 Feb 1;214(Pt 3):353-61. doi: 10.1242/jeb.038588. J Exp Biol. 2011. PMID: 21228194 Free PMC article. Review.
References
-
- Dethier VG. 1989. Patterns of locomotion of polyphagous arctiid caterpillars in relation to foraging. Ecol. Entomol. 14, 375–386. (10.1111/j.1365-2311.1989.tb00939.x) - DOI
-
- Gray J. 1968. Animal locomotion. London: Weidenfeld, Nicolson.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

