Understanding chronic inflammation: couplings between cytokines, ROS, NO, Cai 2+, HIF-1α, Nrf2 and autophagy
- PMID: 40264757
- PMCID: PMC12012389
- DOI: 10.3389/fimmu.2025.1558263
Understanding chronic inflammation: couplings between cytokines, ROS, NO, Cai 2+, HIF-1α, Nrf2 and autophagy
Abstract
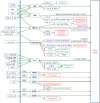
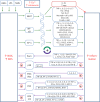
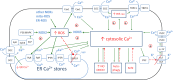
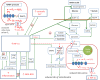
Chronic inflammation is an important component of many diseases, including autoimmune diseases, intracellular infections, dysbiosis and degenerative diseases. An important element of this state is the mainly positive feedback between inflammatory cytokines, reactive oxygen species (ROS), nitric oxide (NO), increased intracellular calcium, hypoxia-inducible factor 1-alpha (HIF-1α) stabilisation and mitochondrial oxidative stress, which, under normal conditions, enhance the response against pathogens. Autophagy and the nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant response are mainly negatively coupled with the above-mentioned elements to maintain the defence response at a level appropriate to the severity of the infection. The current review is the first attempt to build a multidimensional model of cellular self-regulation of chronic inflammation. It describes the feedbacks involved in the inflammatory response and explains the possible pathways by which inflammation becomes chronic. The multiplicity of positive feedbacks suggests that symptomatic treatment of chronic inflammation should focus on inhibiting multiple positive feedbacks to effectively suppress all dysregulated elements including inflammation, oxidative stress, calcium stress, mito-stress and other metabolic disturbances.
Keywords: HIF-1α; NF-κB; autophagy; calcium flux; cytokines; iNOS; inflammation; nitric oxide.
Copyright © 2025 Michalak and Michalak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

