Spinal neuron-glial crosstalk and ion channel dysregulation in diabetic neuropathic pain
- PMID: 40264787
- PMCID: PMC12011621
- DOI: 10.3389/fimmu.2025.1480534
Spinal neuron-glial crosstalk and ion channel dysregulation in diabetic neuropathic pain
Abstract
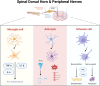
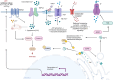
Diabetic neuropathic pain (DNP) is one of the most prevalent complications of diabetes, characterized by a high global prevalence and a substantial affected population with limited effective therapeutic options. Although DNP is closely associated with hyperglycemia, an increasing body of research suggests that elevated blood glucose levels are not the sole inducers of DNP. The pathogenesis of DNP is intricate, involving the release of inflammatory mediators, alterations in synaptic plasticity, demyelination of nerve fibers, and ectopic impulse generation, yet the precise mechanisms remain to be elucidated. The spinal dorsal horn coordinates dynamic interactions between peripheral and central pain pathways, wherein dorsal horn neurons, microglia, and astrocytes synergize with Schwann cell-derived signals to process nociceptive information flow. Abnormally activated neurons can alter signal transduction by modifying the local microenvironment, compromising myelin integrity, and diminishing trophic support, leading to neuronal sensitization and an amplifying effect on peripheral pain signals, which in turn triggers neuropathic pain. Ion channels play a pivotal role in signal conduction, with the modulation of sodium, potassium, and calcium channels being particularly crucial for the regulation of pain signals. In light of the rising incidence of diabetes and the current scarcity of effective DNP treatments, a thorough investigation into the interactions between neurons and glial cells, especially the mechanisms of ion channel function in DNP, is imperative for identifying potential drug targets, developing novel therapeutic strategies, and thereby enhancing the prospects for DNP management.
Keywords: Schwann cells; astrocytes; diabetic neuropathic pain; ion channels; microglia; spinal dorsal horn.
Copyright © 2025 Wu, Hu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Activated microglia in the spinal cord underlies diabetic neuropathic pain.Eur J Pharmacol. 2014 Apr 5;728:59-66. doi: 10.1016/j.ejphar.2014.01.057. Epub 2014 Feb 6. Eur J Pharmacol. 2014. PMID: 24508519 Review.
-
CXCR4/CX43 Regulate Diabetic Neuropathic Pain via Intercellular Interactions between Activated Neurons and Dysfunctional Astrocytes during Late Phase of Diabetes in Rats and the Effects of Antioxidant N-Acetyl-L-Cysteine.Oxid Med Cell Longev. 2022 Jun 28;2022:8547563. doi: 10.1155/2022/8547563. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35799894 Free PMC article.
-
cAMP-PKA signaling is involved in regulation of spinal HCN channels function in diabetic neuropathic pain.Neurosci Lett. 2021 Apr 17;750:135763. doi: 10.1016/j.neulet.2021.135763. Epub 2021 Feb 19. Neurosci Lett. 2021. PMID: 33617945
-
Activation of ephrinB-EphB receptor signalling in rat spinal cord contributes to maintenance of diabetic neuropathic pain.Eur J Pain. 2017 Feb;21(2):278-288. doi: 10.1002/ejp.922. Epub 2016 Jul 27. Eur J Pain. 2017. PMID: 27461472
-
Excitatory and inhibitory neuronal signaling in inflammatory and diabetic neuropathic pain.Mol Med. 2023 Apr 17;29(1):53. doi: 10.1186/s10020-023-00647-0. Mol Med. 2023. PMID: 37069517 Free PMC article. Review.
Cited by
-
Translating Basic Science to Clinical Applications: A Narrative Review of Repurposed Pharmacological Agents in Preclinical Models of Diabetic Neuropathy.Biomedicines. 2025 Jul 13;13(7):1709. doi: 10.3390/biomedicines13071709. Biomedicines. 2025. PMID: 40722780 Free PMC article. Review.
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London England). (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Ceriello A, deValk HW, Guerci B, Haak T, Owens D, Canobbio M, et al. . The burden of type 2 diabetes in Europe: Current and future aspects of insulin treatment from patient and healthcare spending perspectives. Diabetes Res Clin Pract. (2020) 161:108053. doi: 10.1016/j.diabres.2020.108053 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

