Fate plasticity of interneuron specification
- PMID: 40264797
- PMCID: PMC12013500
- DOI: 10.1016/j.isci.2025.112295
Fate plasticity of interneuron specification
Abstract
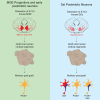
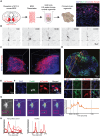
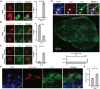
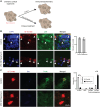
Neuronal subtype generation in the mammalian central nervous system is governed by competing genetic programs. The medial ganglionic eminence (MGE) produces two major cortical interneuron (IN) populations, somatostatin (Sst) and parvalbumin (Pvalb), which develop on different timelines. The extent to which external signals influence these identities remains unclear. Pvalb-positive INs are crucial for cortical circuit regulation but challenging to model in vitro. We grafted mouse MGE progenitors into diverse 2D and 3D co-culture systems, including mouse and human cortical, MGE, and thalamic models. Strikingly, only 3D human corticogenesis models promoted efficient, non-autonomous Pvalb differentiation, characterized by upregulation of Pvalb maturation markers, downregulation of Sst-specific markers, and the formation of perineuronal nets. Additionally, lineage-traced postmitotic Sst-positive INs upregulated Pvalb when grafted onto human cortical models. These findings reveal unexpected fate plasticity in MGE-derived INs, suggesting that their identities can be dynamically shaped by the environment.
Keywords: Biological sciences; Cellular neuroscience; Natural sciences; Neuroscience; Systems neuroscience.
© 2025 The Author(s).
Conflict of interest statement
M.A.M.-R. and A.A.P. are listed as inventors on a patent application concerning the in vitro generation of Pvalb-positive INs. Additionally, M.A.M.-R. and J.L. are inventors on a separate patent application related to machine learning models for neuron classification. L.Z. is an employee of Milecell Biotechnology. D.T. is an employee of Aperture Therapeutics. A.A.-B. and A.R.K. are co-founders and members of the scientific advisory board of Neurona Therapeutics. W.R.M.L. is an employee of Neurona Therapeutics. A.R. is a co-founder and the chief technology officer of Immergo Labs. M.T. is a member of the scientific advisory board of Immergo Labs. M.T. serves as an advisory board member of Open Culture Science, Inc. M.A.M.-R. is an advisor for Atoll Financial Group.
Figures






References
-
- Allison T., Langerman J., Sabri S., Otero-Garcia M., Lund A., Huang J., Wei X., Samarasinghe R.A., Polioudakis D., Mody I., et al. Defining the nature of human pluripotent stem cell-derived interneurons via single-cell analysis. Stem Cell Rep. 2021;16:2548–2564. doi: 10.1016/j.stemcr.2021.08.006. - DOI - PMC - PubMed
-
- Harb K., Richter M., Neelagandan N., Magrinelli E., Harfoush H., Kuechler K., Henis M., Hermanns-Borgmeyer I., Calderon de Anda F., Duncan K. Pum2 and TDP-43 refine area-specific cytoarchitecture post-mitotically and modulate translation of Sox5, Bcl11b, and Rorb mRNAs in developing mouse neocortex. Elife. 2022;11 doi: 10.7554/eLife.55199. - DOI - PMC - PubMed
-
- Nowakowski T.J., Bhaduri A., Pollen A.A., Alvarado B., Mostajo-Radji M.A., Di Lullo E., Haeussler M., Sandoval-Espinosa C., Liu S.J., Velmeshev D., et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science. 2017;358:1318–1323. doi: 10.1126/science.aap8809. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

