Functionalized screen-printed electrodes for non-invasive detection of vascular-endothelial cadherin in extracellular vesicles
- PMID: 40264865
- PMCID: PMC12012609
- DOI: 10.1039/d4ra08926j
Functionalized screen-printed electrodes for non-invasive detection of vascular-endothelial cadherin in extracellular vesicles
Abstract
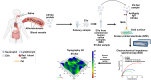
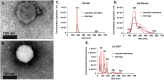
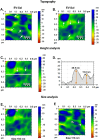
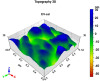
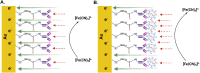
In this study, we developed a biosensor using a gold screen-printed electrode (Au-SPE) functionalized with mercaptoundecanoic acid (MUA) and an antibody for detecting the vascular-endothelial cadherin (CD144) as a endothelial biomarker protein on extracellular vesicles (EVs) isolated from saliva. The MUA functionalization provides a stable platform for immobilizing the CD144 antibody, ensuring the detection of the target protein. This biosensor combines Au-SPE technology with an immunoassay, offering a rapid, sensitive, and non-invasive method for detection of CD144 carried by EVs. Characterization of saliva-derived EVs using transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) confirmed their morphology and size, which fell within the expected range of 80-180 nm. NTA indicated a lower concentration of particles in saliva-EVs than in serum-EVs (controls), highlighting the need for sensitive detection of EV cargos in this type of EV. Immunodetection confirmed the presence of CD144 in both saliva and serum-derived EVs, with higher concentrations in serum. Functionalization of Au-SPEs with MUA and CD144 antibodies was confirmed by significant resistance changes, and atomic force microscopy (AFM) was used to verify the preservation of EV morphology and their capturing post-immune adsorption. A calibration curve demonstrated the high sensitivity of the biosensor prototype for detecting CD144-positive EVs, with a limit of detection (LOD) of 0.111 ng mL-1 and a limit of quantification (LOQ) of 0.37 ng mL-1, requiring only 3 μL of EV-sample. This biosensor shows potential as a novel method for detecting and studying endothelial biomarkers associated with cardiovascular disease in EVs isolated from saliva, a capability not currently available with existing tools. Furthermore, it provides a key platform for expanding research to other biomarkers and diseases by monitoring protein cargos in the EVs, enhancing its utility across diverse clinical applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
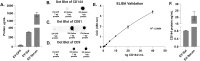


References
-
- Baghdadi N. A. Abdelaliem S. M. F. Malki A. Gad I. Ewis A. Atlam E. J. Big Data. 2023;10:144.
LinkOut - more resources
Full Text Sources
Miscellaneous

