Optimizing algal hydrogen photoproduction: a simplified and efficient protocol for anoxic induction in a semi-autotrophic approach
- PMID: 40265210
- PMCID: PMC12015654
- DOI: 10.1111/ppl.70232
Optimizing algal hydrogen photoproduction: a simplified and efficient protocol for anoxic induction in a semi-autotrophic approach
Abstract
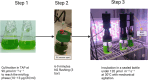
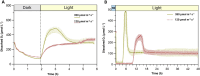
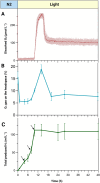
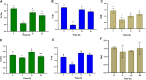
Green microalgae, such as Chlamydomonas reinhardtii, show great potential for producing green hydrogen using only water and sunlight, with no carbon emissions. However, sustainable hydrogen production requires addressing the hydrogenase sensitivity to oxygen and enhancing electron allocation to this enzyme. Previous methods for hydrogen photoproduction rely on a brief nitrogen flushing followed by a dark incubation phase to establish anoxia prior to exposure to high light. In this study, we present a straightforward protocol involving a mixotrophic growth phase followed by a semi-autotrophic hydrogen production phase. During the hydrogen production phase, extended nitrogen flushing induced anoxia in the liquid algae culture, even though oxygen was still present in the headspace. Anoxia was maintained under light at moderate intensity (120 μmol m-2 s-1) and a controlled temperature of 30°C, with an efficient mixing system. Throughout the hydrogen production phase, we monitored dissolved oxygen levels in the culture alongside traditional oxygen measurements in the headspace. Using our protocol with the pgr5 mutant of C. reinhardtii, we achieved a maximum specific rate of 72 μmol H₂ mg-1 Chl h-1 and an average rate of 30-35 μmol H₂ mg-1 Chl h-1 over 10 hours of illumination. Additional nitrogen flushing steps extended anoxia, resulting in a total hydrogen yield of 220 ± 20 mL L-1 over 48 hours of illumination. This performance is attributed to maintaining the redox balance of the plastoquinone pool and minimizing photodamage to the photosystem II complex. Our protocol offers a significant advancement for scalable and sustainable green hydrogen production.
© 2025 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Figures


References
-
- Acién Fernández, F. G. , Fernández Sevilla, J. M. , & Molina Grima, E. (2013). Photobioreactors for the production of microalgae. Reviews in Environmental Science and Biotechnology, 12(2), 131–151
-
- Akkerman, I. , Janssen, M. , Rocha, J. , & Wijffels, R. H. (2002). Photobiological hydrogen production: photochemical efficiency and bioreactor design. International Journal of Hydrogen Energy, 27(112), 1195–1208
-
- Appel, J. , Hueren, V. , Boehm, M. , & Gutekunst, K. (2020). Cyanobacterial in vivo solar hydrogen production using a photosystem I–hydrogenase (PsaD‐HoxYH) fusion complex. Nature Energy, 5(6): 458–467
-
- Benemann, J. R. (1997). Feasibility analysis of photobiological hydrogen production. International Journal of Hydrogen Energy, 22(10–11), 979–987
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

