Metabolic Adaptations and Therapies in Cardiac Hypoxia: Mechanisms and Clinical Implications/ Potential Strategies
- PMID: 40265246
- PMCID: PMC12230486
- DOI: 10.1016/j.jacbts.2024.12.008
Metabolic Adaptations and Therapies in Cardiac Hypoxia: Mechanisms and Clinical Implications/ Potential Strategies
Abstract
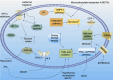
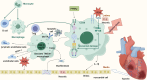
Cardiac hypoxia triggers a cascade of responses and functional changes in myocardial and non-myocardial cells, profoundly affecting cellular metabolism, oxygen-sensing mechanisms, and immune responses. Myocardial cells, being the primary cell type in cardiac tissue, undergo significant alterations in energy metabolism, including glycolysis, fatty acid metabolism, ketone body utilization, and branched-chain amino acid metabolism, to maintain cardiac function under hypoxic conditions. Non-myocardial cells, such as fibroblasts, endothelial cells, and immune cells, although fewer in number, play crucial roles in regulating cardiac homeostasis, maintaining structural integrity, and responding to injury. This review discusses the metabolic reprogramming of immune cells, particularly macrophages, during ischemia-reperfusion injury and explores various therapeutic strategies that modulate these metabolic pathways to protect the heart during hypoxia. Understanding these interactions provides valuable insights and potential therapeutic targets for heart disease treatment.
Keywords: cardiac; cell interaction; hypoxia; metabolism; therapy.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by the National Natural Science Foundation of China (No. 82160157 and No. 81970290), the Joint Funds of the National Natural Science Foundation of China (No. U20A2018), and the Natural Science Foundation of Beijing (No. 7242046 and No. 7222044). Funds by 1·3·5 project for disciplines of excellence (ZYJC21008), West China Hospital, Sichuan University and by CAMS Innovation Fund for Medical Sciences (2019-I2M-5-011,2022-I2M-C&T-B-099). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



Similar articles
-
Hypoxia-induced metabolic reprogramming in mesenchymal stem cells: unlocking the regenerative potential of secreted factors.Front Cell Dev Biol. 2025 Jun 9;13:1609082. doi: 10.3389/fcell.2025.1609082. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40552309 Free PMC article.
-
Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids.Cell Death Dis. 2024 May 1;15(5):308. doi: 10.1038/s41419-024-06703-9. Cell Death Dis. 2024. PMID: 38693114 Free PMC article.
-
MTX2 facilitates PKM2 tetramerization to promote cardiac glucose metabolism and protects the heart against ischemia/reperfusion injury.Theranostics. 2025 Jun 9;15(14):6737-6752. doi: 10.7150/thno.110162. eCollection 2025. Theranostics. 2025. PMID: 40585998 Free PMC article.
-
Relationship between Pituitary Gland and Stem Cell in the Aspect of Hormone Production and Disease Prevention: A Narrative Review.Endocr Metab Immune Disord Drug Targets. 2025;25(7):509-526. doi: 10.2174/0118715303314551241031093717. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39812047 Review.
-
A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury.Front Immunol. 2022 Oct 7;13:930171. doi: 10.3389/fimmu.2022.930171. eCollection 2022. Front Immunol. 2022. PMID: 36275741 Free PMC article.
Cited by
-
Knockout of thyroid hormone receptor alpha a (thraa) enhances cardiac regeneration in zebrafish through metabolic and hypoxic regulation.Cell Commun Signal. 2025 Jul 16;23(1):340. doi: 10.1186/s12964-025-02350-5. Cell Commun Signal. 2025. PMID: 40671086 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

