Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study
- PMID: 40265382
- PMCID: PMC12015901
- DOI: 10.3390/medsci13020035
Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study
Abstract
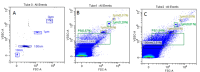
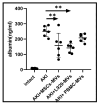
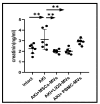
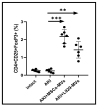
Background/Objectives: This investigation compared the therapeutic efficacy of extracellular microvesicles (MVs) derived from murine L929 sarcoma cells and murine mesenchymal stem cells (MSCs). Methods: A mouse model of acute kidney injury (AKI) was used. Results: Both MVs from L929 cells (L929-MVs) and MSCs (MSC-MVs), unlike those obtained from murine peripheral blood mononuclear cells (PBMCs), enhanced survival rates in AKI mice and significantly improved kidney function. This was indicated by decreased levels of urine albumin and serum creatinine. Furthermore, treatment with L929-MVs and MSC-MVs elevated the proportions of CD4+CD25+FOXP3+ regulatory T cells while reducing the presence of pro-inflammatory CD4+CD44+ T cells in the spleens of AKI mice. Conclusions: the results highlight the potential of tumour-derived MVs to facilitate organ repair and exert cytoprotective immunomodulatory effects.
Keywords: acute kidney injury; mesenchymal stem cell; microvesicles; regeneration; tumour.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury.PLoS One. 2012;7(3):e33115. doi: 10.1371/journal.pone.0033115. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22431999 Free PMC article.
-
Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury.Nephrol Dial Transplant. 2011 May;26(5):1474-83. doi: 10.1093/ndt/gfr015. Epub 2011 Feb 15. Nephrol Dial Transplant. 2011. PMID: 21324974
-
Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice.Am J Respir Crit Care Med. 2015 Aug 1;192(3):324-36. doi: 10.1164/rccm.201410-1765OC. Am J Respir Crit Care Med. 2015. PMID: 26067592 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges.Stem Cell Res Ther. 2017 Dec 4;8(1):273. doi: 10.1186/s13287-017-0727-7. Stem Cell Res Ther. 2017. PMID: 29202871 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury.Front Immunol. 2021 Jun 3;12:684496. doi: 10.3389/fimmu.2021.684496. eCollection 2021. Front Immunol. 2021. PMID: 34149726 Free PMC article. Review.
References
-
- Kumar M.A., Baba S.K., Sadida H.Q., Marzooqi S.A., Jerobin J., Altemani F.H., Algehainy N., Alanazi M.A., Abou-Samra A.B., Kumar R., et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024;9:27. doi: 10.1038/s41392-024-01735-1. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

