Teichoic acids in the periplasm and cell envelope of Streptococcus pneumoniae
- PMID: 40265569
- PMCID: PMC12017771
- DOI: 10.7554/eLife.105132
Teichoic acids in the periplasm and cell envelope of Streptococcus pneumoniae
Abstract
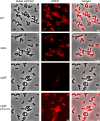
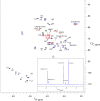
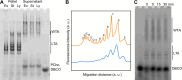
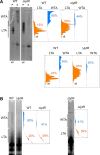
Teichoic acids (TA) are linear phospho-saccharidic polymers and important constituents of the cell envelope of Gram-positive bacteria, either bound to the peptidoglycan as wall teichoic acids (WTA) or to the membrane as lipoteichoic acids (LTA). The composition of TA varies greatly but the presence of both WTA and LTA is highly conserved, hinting at an underlying fundamental function that is distinct from their specific roles in diverse organisms. We report the observation of a periplasmic space in Streptococcus pneumoniae by cryo-electron microscopy of vitreous sections. The thickness and appearance of this region change upon deletion of genes involved in the attachment of TA, supporting their role in the maintenance of a periplasmic space in Gram-positive bacteria as a possible universal function. Consequences of these mutations were further examined by super-resolved microscopy, following metabolic labeling and fluorophore coupling by click chemistry. This novel labeling method also enabled in-gel analysis of cell fractions. With this approach, we were able to titrate the actual amount of TA per cell and to determine the ratio of WTA to LTA. In addition, we followed the change of TA length during growth phases, and discovered that a mutant devoid of LTA accumulates the membrane-bound polymerized TA precursor.
Keywords: Streptococcus pneumoniae; bacterial cell wall; biochemistry; chemical biology; infectious disease; microbiology; periplasm; teichoic acid.
© 2025, Nguyen, Bauda et al.
Conflict of interest statement
MN, EB, CB, CL, CF, AC, BG, MB, YW, CG, CM, CD, AZ, CM No competing interests declared
Figures
















Update of
- doi: 10.1101/2024.10.18.619035
- doi: 10.7554/eLife.105132.1
- doi: 10.7554/eLife.105132.2
References
-
- Badger E. The structural specificity of choline for the growth of type III pneumococcus. Journal of Biological Chemistry. 1944;153:183–191. doi: 10.1016/S0021-9258(18)51225-4. - DOI
-
- Barreteau H, Bouhss A, Fourgeaud M, Mainardi J-L, Touzé T, Gérard F, Blanot D, Arthur M, Mengin-Lecreulx D. Human- and plant-pathogenic Pseudomonas species produce bacteriocins exhibiting colicin M-like hydrolase activity towards peptidoglycan precursors. Journal of Bacteriology. 2009;191:3657–3664. doi: 10.1128/JB.01824-08. - DOI - PMC - PubMed
-
- Barreteau H, Tiouajni M, Graille M, Josseaume N, Bouhss A, Patin D, Blanot D, Fourgeaud M, Mainardi J-L, Arthur M, van Tilbeurgh H, Mengin-Lecreulx D, Touzé T. Functional and structural characterization of PaeM, a colicin M-like bacteriocin produced by Pseudomonas aeruginosa. The Journal of Biological Chemistry. 2012;287:37395–37405. doi: 10.1074/jbc.M112.406439. - DOI - PMC - PubMed
-
- Bauda E, Gallet B, Moravcova J, Effantin G, Chan H, Novacek J, Jouneau P-H, Rodrigues CDA, Schoehn G, Moriscot C, Morlot C. Ultrastructure of macromolecular assemblies contributing to bacterial spore resistance revealed by in situ cryo-electron tomography. Nature Communications. 2024;15:1376. doi: 10.1038/s41467-024-45770-6. - DOI - PMC - PubMed
-
- Bonnet J, Durmort C, Mortier-Barrière I, Campo N, Jacq M, Moriscot C, Straume D, Berg KH, Håvarstein L, Wong YS, Vernet T, Di Guilmi AM. Nascent teichoic acids insertion into the cell wall directs the localization and activity of the major pneumococcal autolysin LytA. Cell Surface. 2018a;2:24–37. doi: 10.1016/j.tcsw.2018.05.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

