Dual roles of influenza B virus neuraminidase mRNA vaccine in enhancing cross-lineage protection by supplementing inactivated split vaccination
- PMID: 40265888
- PMCID: PMC12090766
- DOI: 10.1128/jvi.02294-24
Dual roles of influenza B virus neuraminidase mRNA vaccine in enhancing cross-lineage protection by supplementing inactivated split vaccination
Abstract
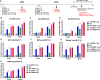
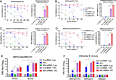
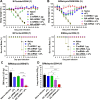
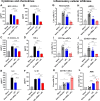
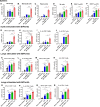
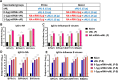
The current influenza vaccine is based on immunity to hemagglutinin (HA) and provides poor cross-protection. Here, we generated mRNA vaccine encoding influenza B virus (IBV) neuraminidase (NA) conjugated to influenza A virus M2 ectodomain (M2e), encapsulated in lipid nanoparticles (LNP), capable of inducing cross-lineage IBV protection in a dose-dependent pattern. The combination of low-dose NA mRNA and inactivated split IBV vaccines was found to induce significantly higher levels of cross-reactive IgG responses, NA and HA inhibition titers, effector and memory cellular immune responses as well as cross-lineage protection than either NA mRNA or split vaccine alone. This study suggests that the NA mRNA vaccine not only provides cross-lineage protection with a high dose but also enhances the cross-protective efficacy of the combined low-dose NA mRNA and split vaccines. Our findings support a new strategy of using mRNA LNP-supplemented conventional vaccination to enhance cross-protection.IMPORTANCEThis study highlights a significant advancement in influenza vaccination strategies. To test a new vaccination strategy, we developed an influenza B virus (IBV) neuraminidase (NA) mRNA vaccine which could provide cross-lineage protection at a high dose. More importantly, the co-administration of NA mRNA and split IBV vaccine at low doses was found to significantly enhance the hemagglutinin and NA immunity as well as cross-lineage protection of seasonal IBV vaccines. This proof-of-concept study provides evidence for a novel strategy to enhance the immunogenicity and cross-protective efficacy of conventional vaccines by supplementing with new targets of mRNA vaccines.
Keywords: NA mRNA-LNP; cross-protection; influenza virus; split IBV vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gravenstein S, Davidson HE, Taljaard M, Ogarek J, Gozalo P, Han L, Mor V. 2017. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 5:738–746. doi:10.1016/S2213-2600(17)30235-7 - DOI - PubMed
-
- Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, Monto AS, McLean HQ, Belongia EA, Gaglani M, Murthy K, Fry AM, Flannery B, Investigators U, Investigators USFV. 2016. 2014-2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 63:1564–1573. doi:10.1093/cid/ciw635 - DOI - PMC - PubMed
-
- Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F. 2015. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 6:e02556. doi:10.1128/mBio.02556-14 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

