EGF-Induced Macropinocytosis Promotes NAV1-Dependent Internalization of Occludin in Keratinocytes
- PMID: 40266031
- PMCID: PMC12017258
- DOI: 10.1096/fj.202402876R
EGF-Induced Macropinocytosis Promotes NAV1-Dependent Internalization of Occludin in Keratinocytes
Abstract
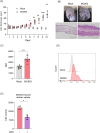
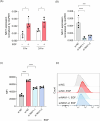
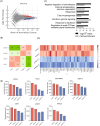
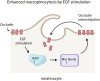
Epidermal keratinocytes form the outermost layer of the skin and serve as a pivotal barrier against external insults. This barrier, however, can be compromised in conditions such as atopic dermatitis (AD), where both genetic and environmental factors contribute to its disruption. Recent studies have indicated that macropinocytosis, a non-selective endocytic process, is involved in the internalization of barrier proteins. In this study, we explored the role of macropinocytosis in differentiated keratinocytes and its potential impact on skin barrier integrity in AD. Our results demonstrated that epidermal growth factor (EGF), but not the type 2 cytokines IL-4 and IL-13, significantly promoted macropinocytosis in differentiated HaCaT keratinocytes. EGF stimulation increased the uptake of 70 kDa dextran and induced the internalization of occludin, a component of tight junction proteins. Furthermore, enhanced macropinocytosis was observed in the epidermis of a mouse model of AD, accompanied by elevated EGF expression in the skin, indicating that the AD skin microenvironment may drive this process. NAV1 was identified as a critical regulator of EGF-induced macropinocytosis, as its knockdown significantly impaired this process. Transcriptome analysis of NAV1-knockdown cells further revealed changes in the expression of Rho family GTPases, including CDC42 and MMP14, suggesting that NAV1 modulates macropinocytosis through Rho-dependent pathways. These findings provide new insights into the regulation of macropinocytosis in keratinocytes and its potential contribution to the barrier dysfunction observed in AD.
Keywords: atopic; dermatitis; endocytosis; rho GTP‐binding proteins; tight junction proteins.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ständer S., “Atopic Dermatitis,” New England Journal of Medicine 384 (2021): 1136–1143. - PubMed
-
- Drislane C. and Irvine A. D., “The Role of Filaggrin in Atopic Dermatitis and Allergic Disease,” Annals of Allergy, Asthma & Immunology 124 (2020): 36–43. - PubMed
-
- Koga C., Kabashima K., Shiraishi N., Kobayashi M., and Tokura Y., “Possible Pathogenic Role of Th17 Cells for Atopic Dermatitis,” Journal of Investigative Dermatology 128 (2008): 2625–2630. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

