Efficacy of a Self-Vaccination Strategy for Influenza A Virus, Mycoplasma hyopneumoniae, Erysipelothrix rhusiopathiae, and Lawsonia intracellularis in Swine
- PMID: 40266075
- PMCID: PMC11946863
- DOI: 10.3390/vaccines13030229
Efficacy of a Self-Vaccination Strategy for Influenza A Virus, Mycoplasma hyopneumoniae, Erysipelothrix rhusiopathiae, and Lawsonia intracellularis in Swine
Abstract
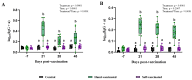
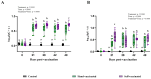
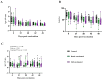
Background/Objectives: Environmental enrichment (EE) devices are required in various countries and markets to promote animal welfare, with dual-purpose devices more likely to encourage adoption. We developed an EE device that allows pigs to self-administer liquids, designed to align with natural and play behaviors, and utilized a maternal pheromone (MP) to attract pigs to the device. This study aimed to evaluate the efficacy of this device in delivering vaccines for Erysipelas, Ileitis, Mycoplasma, and Influenza to growing pigs. Methods: Pigs were assigned to three treatments groups: Control (unvaccinated), Hand-Vaccinated (via oral gavage or intramuscular injection), and Self-Vaccinated using the EE device. Baseline samples were collected to determine initial antibody status, and serum and oral fluids' IgG and IgA levels were measured post-vaccination to assess immune response. Four studies were conducted with 36 pigs (12 per treatment) over a 49-day period. Results: Self-vaccination pigs receiving the avirulent live Erysipelas vaccine developed oral and serum antibodies comparable to Hand-Vaccinated pigs. Pigs self-administering the avirulent live Lawsonia intracelluaris vaccine developed oral fluid antibodies. In contrast, pigs who received Mycoplasma or Influenza vaccines through self-vaccination exhibited significantly lower antibody levels compared to the Hand-Vaccinated group. Conclusions: These findings demonstrated that self-vaccination using EE devices for the oral administration of avirulent live vaccines offers benefits such as reduced labor and improved animal welfare. However, killed vaccines did not elicit sufficient antibody responses, suggesting the need for modified vaccine formulations or administration strategies to improve self-vaccination efficacy.
Keywords: behavior; environmental enrichment; pigs; self-vaccination.
Conflict of interest statement
The corresponding author declares a conflict of interest in that he is listed as the inventor of patents (owned by Texas Tech University) for the maternal pheromone and the EE sprayer. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. Author Rebecca Robbins was employed by the company Pig Improvement Company (PIC). Author Jessica Seate was employed by the company Animal Science Products. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Efficacy of a novel inactivated Lawsonia intracellularis vaccine in pigs against experimental infection and under field conditions.Vaccine. 2019 Apr 3;37(15):2149-2157. doi: 10.1016/j.vaccine.2019.02.067. Epub 2019 Mar 11. Vaccine. 2019. PMID: 30867100
-
Systemic and local immune response in pigs intradermally and intramuscularly injected with inactivated Mycoplasma hyopneumoniae vaccines.Vet Microbiol. 2014 Jan 31;168(2-4):357-64. doi: 10.1016/j.vetmic.2013.11.025. Epub 2013 Nov 28. Vet Microbiol. 2014. PMID: 24332702 Clinical Trial.
-
Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant.Aust Vet J. 1997 Jul;75(7):504-11. doi: 10.1111/j.1751-0813.1997.tb14383.x. Aust Vet J. 1997. PMID: 9258425
-
Self-administration of a Salmonella vaccine by domestic pigs.Sci Rep. 2023 Feb 20;13(1):2972. doi: 10.1038/s41598-023-29987-x. Sci Rep. 2023. PMID: 36806288 Free PMC article.
-
Well-Being and Performance of Nursery Pigs Subjected to Different Commercial Vaccines Against Porcine Circovirus Type 2, Mycoplasma hyopneumoniae and Lawsonia intracellularis.Vaccines (Basel). 2024 Oct 31;12(11):1242. doi: 10.3390/vaccines12111242. Vaccines (Basel). 2024. PMID: 39591145 Free PMC article.
References
-
- Selke M., Meens J., Springer S., Frank R., Gerlach G.-F. Immunization of Pigs To Prevent Disease in Humans: Construction and Protective Efficacy of a Salmonella Enterica Serovar Typhimurium Live Negative-Marker Vaccine. Infect. Immun. 2007;75:2476–2483. doi: 10.1128/IAI.01908-06. - DOI - PMC - PubMed
-
- Boessen C., Artz G., Schulz L., Cook H. A Baseline Study of Labor Issues and Trends in U.S. Pork Production. National Pork Producers Council; Urbandale, IA, USA: 2018.
-
- Dubman R. Agricultural Income and Finance Situation and Outlook: 2021 Edition. United States Department of Agriculture (USDA); Washington, DC, USA: 2021. - DOI
-
- Chase C.C.L., Daniels C.S., Garcia R., Milward F., Nation T. Needle-Free Injection Technology in Swine: Progress toward Vaccine Efficacy and Pork Quality. J. Swine Health Prod. 2008;16:254–261. doi: 10.54846/jshap/555. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

