Active Immunization Using TRPM2 Peptide Vaccine Attenuates Atherosclerotic Progression in a Mouse Model of Atherosclerosis
- PMID: 40266108
- PMCID: PMC11946763
- DOI: 10.3390/vaccines13030241
Active Immunization Using TRPM2 Peptide Vaccine Attenuates Atherosclerotic Progression in a Mouse Model of Atherosclerosis
Abstract
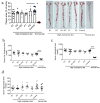
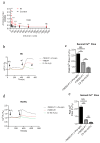
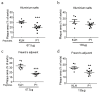
Background/Objective: Atherosclerosis is one of the leading causes of cardiovascular diseases and mortality around the world. One exciting strategy for atherosclerosis treatment is immunotherapy, especially active immunization. Active immunization relies on the delivery of antigens in a vaccine platform to introduce humoral and cellular immunity, alleviating atherosclerotic progression. Transient receptor potential channel isoform M2 (TRPM2) is an ROS-activated Ca2+-permeable ion channel that can promote atherosclerosis via stimulating vascular inflammation. In the present study, we developed a strategy of active immunization with the TRPM2 E3 domain peptide in a vaccine platform, aiming to induce the endogenous production of anti-TRPM2 blocking antibody in mice in vivo, consequently inhibiting TRPM2 channel activity to alleviate atherosclerotic progression. Methods: ApoE knockout mice were fed with a high cholesterol diet to develop atherosclerosis. The mice were injected with or without the E3 peptide vaccines, followed by analysis of atherosclerotic lesion by en face Oil Red O staining of the whole aorta and histologic analysis of thin tissue sections from aortic roots. Results: The results show that immunization with a pig TRPM2 E3 region-based peptide (P1) could effectively alleviate high cholesterol diet-induced atherosclerosis in ApoE knockout mice. We worked out the best vaccine formulation for the most effective atheroprotection, namely P1 at the dose of 67.5 µg per mouse (2.5 mg/kg body weight) with aluminum salts as adjuvant. Conclusions: The present study provides a novel target TRPM2 for peptide vaccine-based anti-atherosclerotic strategy and lays the foundation for future preclinical/clinical trials using TRPM2 E3 P1 vaccine for a potential therapeutic option against atherosclerosis.
Keywords: TRPM2; atherosclerosis; immunization; peptide vaccine.
Conflict of interest statement
Author Xiao Li was employed by the company Inner Mongolia Mengniu Dairy (Group) Co., Ltd. The remaining authors declare no conflicts of interest.
Figures



Similar articles
-
TRPM2 Promotes Atherosclerotic Progression in a Mouse Model of Atherosclerosis.Cells. 2022 Apr 22;11(9):1423. doi: 10.3390/cells11091423. Cells. 2022. PMID: 35563730 Free PMC article.
-
The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice.Eur Heart J. 2017 Aug 21;38(32):2499-2507. doi: 10.1093/eurheartj/ehx260. Eur Heart J. 2017. PMID: 28637178 Free PMC article.
-
Macrophage Foam Cell-Targeting Immunization Attenuates Atherosclerosis.Front Immunol. 2019 Jan 10;9:3127. doi: 10.3389/fimmu.2018.03127. eCollection 2018. Front Immunol. 2019. PMID: 30687328 Free PMC article.
-
Transient Receptor Potential Melastatin 2 Enhances Vascular Reactivity During Development of Atherosclerosis in Mice.Am J Hypertens. 2021 Feb 18;34(1):121-122. doi: 10.1093/ajh/hpaa154. Am J Hypertens. 2021. PMID: 33599748
-
The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation.Immunol Res. 2013 Mar;55(1-3):241-8. doi: 10.1007/s12026-012-8373-8. Immunol Res. 2013. PMID: 22975787 Review.
References
-
- Roquer J., Ois A. Atherosclerotic burden and mortality. In: Preedy V.R., Watson R.R., editors. Handbook of Disease Burdens and Quality of Life Measures. Springer; New York, NY, USA: 2010.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

