Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses
- PMID: 40266196
- PMCID: PMC11946197
- DOI: 10.3390/vaccines13030311
Inflammatory and Humoral Immune Responses to Commercial Autogenous Salmonella Bacterin Vaccines in Light-Brown Leghorn Pullets: Primary and Secondary Vaccine Responses
Abstract
Background/objectives: Commercial poultry flocks undergo Salmonella vaccinations to manage salmonellosis outbreaks. Due to reports of severe injection site reactions to Salmonella bacterins, assessment of local inflammatory responses is necessary. The objective was to assess local inflammatory and systemic humoral immune responses to commercial autogenous Salmonella bacterin vaccines (SV1 or SV2) following primary or secondary intradermal (i.d.) vaccination in Light-Brown Leghorns (LBLs).
Methods: LBL pullets received primary (14 wks) or secondary (19 wks) vaccination by i.d. growing feather (GF) pulp injection of SV1, SV2, Salmonella Enteritidis (SE) lipopolysaccharide (LPS), or water-oil-water emulsion (V). Local leukocyte levels and relative cytokine mRNA expression were monitored before (0 d) and at 6 h, 1 d, 2 d, 3 d, 5 d, and 7 d post-GF pulp injection (p.i.). Blood was collected through 28 d post-primary or -secondary vaccination, and SE-specific antibodies were quantified via ELISA.
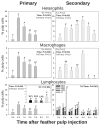
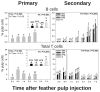
Results: Primary vaccine administration increased local heterophil and macrophage levels and increased IL-6 and IL-8 mRNA expressions at 6 h p.i., independent of treatment. Secondary administration extended these local immune activities through 3 d p.i. and included prolonged IL-17A mRNA expression. Primary and secondary GF-pulp injection with V resulted in rapid lymphocyte recruitment by 6 h p.i., comprised primarily of CD4+ and γδ T cells. SV1 and SV2 also produced a T-dependent systemic humoral immune response, as indicated by the IgM-to-IgG isotype switch, along with a memory phenotype in the secondary response.
Conclusions: These commercial-killed Salmonella vaccines, when prepared in water-oil-water emulsions, stimulated prolonged innate and T helper (Th) 17-type inflammatory responses at the injection site and produced a classic systemic humoral immune response after a second vaccination. Further research is needed to determine if extended inflammation influences adaptive immune responses in eliminating Salmonella infection.
Keywords: Salmonella vaccine; T cells; antibody; chicken; inflammation; innate immunity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Local Inflammatory and Systemic Antibody Responses Initiated by a First Intradermal Administration of Autogenous Salmonella-Killed Vaccines and Their Components in Pullets.Vaccines (Basel). 2024 Oct 11;12(10):1159. doi: 10.3390/vaccines12101159. Vaccines (Basel). 2024. PMID: 39460325 Free PMC article.
-
Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram- and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine.Vet Sci. 2025 Mar 16;12(3):279. doi: 10.3390/vetsci12030279. Vet Sci. 2025. PMID: 40266996 Free PMC article.
-
Bovine Immune Response to Vaccination and Infection with Leptospira borgpetersenii Serovar Hardjo.mSphere. 2021 Mar 24;6(2):e00988-20. doi: 10.1128/mSphere.00988-20. mSphere. 2021. PMID: 33762318 Free PMC article.
-
Humoral and cellular immune response generated by different vaccine programs before and after Salmonella Enteritidis challenge in chickens.Vaccine. 2012 Dec 14;30(52):7637-43. doi: 10.1016/j.vaccine.2012.10.020. Epub 2012 Oct 18. Vaccine. 2012. PMID: 23085366
-
The immunobiology of avian systemic salmonellosis.Vet Immunol Immunopathol. 2009 Mar 15;128(1-3):53-9. doi: 10.1016/j.vetimm.2008.10.295. Epub 2008 Oct 17. Vet Immunol Immunopathol. 2009. PMID: 19070366 Review.
References
-
- Hy-Line International Vaccination Recommendations. [(accessed on 4 May 2021)]. Available online: https://www.hyline.com/ViewFile?id=7c275f35-64c0-466a-a40f-eb24d1fe0d9f.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

