Protection Against Pneumonia Induced by Vaccination with Fimbriae Subunits from Klebsiella pneumoniae
- PMID: 40266206
- PMCID: PMC11945627
- DOI: 10.3390/vaccines13030303
Protection Against Pneumonia Induced by Vaccination with Fimbriae Subunits from Klebsiella pneumoniae
Abstract
Background: Klebsiella pneumoniae infections pose a great burden worldwide, causing high morbidity and mortality, which are worsened by the increase in multidrug-resistant strains. New therapeutic/prophylactic strategies are urgently needed to overcome antibiotic resistance and reduce the health and economic impacts of diseases caused by this pathogen. Fimbriae are important virulence factors involved in biofilm formation and adhesion to host cells. Their exposed location, conservation among clinical isolates and adjuvant properties make them interesting candidates for inclusion in protein-based vaccines. Therefore, the present work investigated the immunological potential of type 1 and 3 fimbriae subunits in a murine model of K. pneumoniae lung infection.
Methods: MrkA and FimA were produced as recombinant proteins in E. coli, purified and used to immunize mice subcutaneously. The immune responses were characterized and protection against pneumonia was evaluated after intranasal challenge.
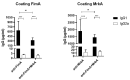
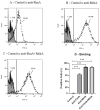
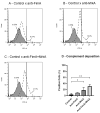
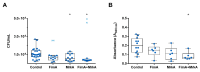
Results: Subcutaneous immunization with recombinant FimA and MrkA induced high IgG1 production; the antibodies efficiently recognized the native proteins at the bacterial surface, promoted C3 deposition and reduced biofilm formation by K. pneumoniae in vitro. Mice vaccinated with the co-administered proteins reduced the bacterial loads in the lungs after intranasal challenge, less inflammation and reduced tissue damage.
Conclusion: The results suggest that both type 1 and type 3 fimbriae contribute to protection against K. pneumoniae lung infection, inducing antibodies that bind to the bacteria and favoring Complement deposition and clearance by the host, while inhibiting biofilm formation.
Keywords: K. pneumoniae; biofilms; fimbriae; protein vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In-silico and experimental analysis of Klebsiella pneumoniae fimbriae subunits for vaccine development.Vaccine. 2025 Apr 19;53:127075. doi: 10.1016/j.vaccine.2025.127075. Epub 2025 Apr 11. Vaccine. 2025. PMID: 40203594
-
Characterization of mAbs against Klebsiella pneumoniae type 3 fimbriae isolated in a target-independent phage display campaign.Microbiol Spectr. 2024 Aug 6;12(8):e0040024. doi: 10.1128/spectrum.00400-24. Epub 2024 Jun 28. Microbiol Spectr. 2024. PMID: 38940542 Free PMC article.
-
Immunoprotective efficacy of 3 Klebsiella pneumoniae type I fimbriae proteins in a murine model.Vet Microbiol. 2024 Oct;297:110197. doi: 10.1016/j.vetmic.2024.110197. Epub 2024 Jul 24. Vet Microbiol. 2024. PMID: 39126781
-
Regulation of biofilm formation in Klebsiella pneumoniae.Front Microbiol. 2023 Sep 7;14:1238482. doi: 10.3389/fmicb.2023.1238482. eCollection 2023. Front Microbiol. 2023. PMID: 37744914 Free PMC article. Review.
-
Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation.Future Microbiol. 2012 Aug;7(8):991-1002. doi: 10.2217/fmb.12.74. Future Microbiol. 2012. PMID: 22913357 Review.
Cited by
-
Engineering and Evaluation of a Live-Attenuated Vaccine Candidate with Enhanced Type 1 Fimbriae Expression to Optimize Protection Against Salmonella Typhimurium.Vaccines (Basel). 2025 Jun 19;13(6):659. doi: 10.3390/vaccines13060659. Vaccines (Basel). 2025. PMID: 40573990 Free PMC article.
References
-
- WHO . Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Volume 72 World Health Organization; Geneva, Switzerland: 2024.
-
- Tan Y.H., Arros P., Berrios-Pasten C., Wijaya I., Chu W.H.W., Chen Y., Cheam G., Mohamed Naim A.N., Marcoleta A.E., Ravikrishnan A., et al. Hypervirulent Klebsiella pneumoniae employs genomic island encoded toxins against bacterial competitors in the gut. ISME J. 2024;18:wrae143. doi: 10.1093/ismejo/wrae054. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

