Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods
- PMID: 40266209
- PMCID: PMC11946853
- DOI: 10.3390/vaccines13030304
Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods
Abstract
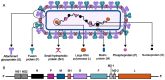
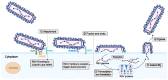
Respiratory syncytial virus (RSV) remains a significant global health threat, especially to infants, the elderly, and immunocompromised individuals. This review comprehensively explores the progress in RSV vaccine development, the immune evaluation methods, and immunological surrogate. The RSV fusion (F) protein, a primary target for vaccine development, has been engineered in prefusion conformation to elicit potent neutralizing antibodies, while the attachment (G) glycoprotein and other immunogens are also being explored to broaden immune responses. Advances in diverse vaccine platforms, ranging from live attenuated and protein subunit vaccines to cutting-edge mRNA- and nanoparticle-based formulations, highlight the field's progress, yet challenges in balancing safety, immunogenicity, and durability persist. Central to these efforts is the identification and validation of immunological surrogates, which may serve as critical benchmarks for vaccine efficacy. Neutralizing antibody titers, multifunctional T cell responses, and B cell memory have emerged as key correlates of protection. However, the feasibility of these surrogates depends on their ability to predict clinical outcomes across diverse populations and settings. While neutralizing antibodies block the virus directly, T cell responses are essential for clearing infected cells and preventing severe disease, and B cell memory ensures long-term immunity. Integrating these immunological markers into a cohesive framework requires standardized assays, robust clinical validation, and an in-depth understanding of RSV-induced immune response.
Keywords: immunological surrogates; respiratory syncytial virus; vaccine evaluation; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
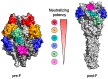

Similar articles
-
A Parainfluenza Virus Vector Expressing the Respiratory Syncytial Virus (RSV) Prefusion F Protein Is More Effective than RSV for Boosting a Primary Immunization with RSV.J Virol. 2020 Dec 22;95(2):e01512-20. doi: 10.1128/JVI.01512-20. Print 2020 Dec 22. J Virol. 2020. PMID: 33115876 Free PMC article.
-
The Respiratory Syncytial Virus (RSV) G Protein Enhances the Immune Responses to the RSV F Protein in an Enveloped Virus-Like Particle Vaccine Candidate.J Virol. 2023 Jan 31;97(1):e0190022. doi: 10.1128/jvi.01900-22. Epub 2023 Jan 5. J Virol. 2023. PMID: 36602367 Free PMC article.
-
Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: Structure, antigenic profile, immunogenicity, and protection.Vaccine. 2019 Sep 24;37(41):6112-6124. doi: 10.1016/j.vaccine.2019.07.089. Epub 2019 Aug 12. Vaccine. 2019. PMID: 31416644
-
The third pandemic: The respiratory syncytial virus landscape and specific considerations for the allergist/immunologist.Allergy Asthma Proc. 2023 Jul 26;44(4):220-228. doi: 10.2500/aap.2023.44.230030. Allergy Asthma Proc. 2023. PMID: 37236777 Review.
-
RSV neutralization assays - Use in immune response assessment.Vaccine. 2021 Jul 30;39(33):4591-4597. doi: 10.1016/j.vaccine.2021.06.016. Epub 2021 Jul 6. Vaccine. 2021. PMID: 34244007 Review.
Cited by
-
Advancing mRNA vaccines for infectious diseases: key components, innovations, and clinical progress.Essays Biochem. 2025 May 1;69(2):EBC20253009. doi: 10.1042/EBC20253009. Essays Biochem. 2025. PMID: 40321006 Free PMC article. Review.
References
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Higdon M.M., Le T., O’Brien K.L., Murdoch D.R., Prosperi C., Baggett H.C., Brooks W.A., Feikin D.R., Hammitt L.L., Howie S.R.C., et al. Association of C-Reactive Protein With Bacterial and Respiratory Syncytial Virus-Associated Pneumonia Among Children Aged <5 Years in the PERCH Study. Clin. Infect. Dis. 2017;64((Suppl. 3)):S378–S386. doi: 10.1093/cid/cix150. - DOI - PMC - PubMed
-
- Savic M., Penders Y., Shi T., Branche A., Pircon J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses. 2023;17:e13031. doi: 10.1111/irv.13031. - DOI - PMC - PubMed
-
- Kapikian A.Z., Mitchell R.H., Chanock R.M., Shvedoff R.A., Stewart C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

