Immunogenicity and Safety of the Bivalent Respiratory Syncytial Virus Prefusion F Subunit Vaccine in Immunocompromised or Renally Impaired Adults
- PMID: 40266222
- PMCID: PMC11946143
- DOI: 10.3390/vaccines13030328
Immunogenicity and Safety of the Bivalent Respiratory Syncytial Virus Prefusion F Subunit Vaccine in Immunocompromised or Renally Impaired Adults
Abstract
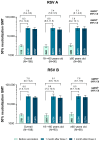
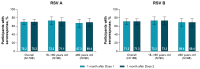
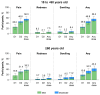
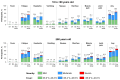
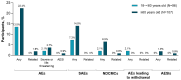
Background/Objectives: Individuals with immunocompromising conditions are at high risk of developing severe respiratory syncytial virus (RSV) illness. This phase 3, single-arm study assessed the safety and immunogenicity of the bivalent RSV prefusion F protein-based (RSVpreF) 120-µg vaccine in immunocompromised and renally impaired adults. Methods: Participants were stratified by age group (18-<60-year-olds; ≥60-year-olds) and received two RSVpreF doses 1 month apart (i.e., Dose 1 and Dose 2, respectively). Reactogenicity events were collected for 7 days after each dose; adverse events through 1 month after the last dose; and serious adverse events, adverse events of special interest, and newly diagnosed chronic medical conditions throughout the study. Results: One month after Dose 1, RSVpreF elicited robust immune responses overall and across age and immunocompromised subgroups. Overall, geometric mean fold rises from before to 1 month after Dose 1 were high for RSV A and RSV B (8.3 and 9.0, respectively); no additional increases 1 month after Dose 2 (7.5 and 7.8) were observed. The most frequent local reaction was pain at the injection site, which was more common after Dose 2 than after Dose 1. The most frequent systemic event after any dose was fatigue. Most local reactions and systemic events were mild or moderate in severity. Adverse event and serious adverse event rates were 13.5% and 7.3% among 18-<60-year-olds and 22.4% and 14.0% among ≥60-year-olds, respectively. Conclusions: A single dose of the RSVpreF vaccine conferred robust immune responses in immunocompromised and renally impaired adults with no safety concerns. (ClinicalTrials.gov Identifier: NCT05842967).
Keywords: RSV; RSVpreF; adults; immunocompromised; immunogenicity; renally impaired; safety.
Conflict of interest statement
Natalia Castillo Almeida, Elissa Malkin, Matthew Davis, and Kapil Saharia have no conflicts to declare. Lalitha Parameswaran received salary support from Robert A Winn Career Development Program, contractual support from Pfizer, Sanofi, and PATH. William J. Towner declared research grants paid to institution by Pfizer, Moderna, Janssen, AstraZeneca, ViiV, Gilead, Merck, and Lumen. All other authors are employees of Pfizer Inc and may hold stock or stock options.
Figures

References
-
- Nguyen-Van-Tam J.S., O’Leary M., Martin E.T., Heijnen E., Callendret B., Fleischhackl R., Comeaux C., Tran T.M.P., Weber K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022;31:220105. doi: 10.1183/16000617.0105-2022. - DOI - PMC - PubMed
-
- Chatzis O., Darbre S., Pasquier J., Meylan P., Manuel O., Aubert J.D., Beck-Popovic M., Masouridi-Levrat S., Ansari M., Kaiser L., et al. Burden of severe RSV disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect. Dis. 2018;18:111. doi: 10.1186/s12879-018-3002-3. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

