Optimizing Tildrakizumab Dosing in Psoriasis: A 52-Week Multicenter Retrospective Study Comparing 100 mg and 200 mg-IL PSO (Italian Landscape Psoriasis)
- PMID: 40266488
- PMCID: PMC12092844
- DOI: 10.1007/s13555-025-01416-z
Optimizing Tildrakizumab Dosing in Psoriasis: A 52-Week Multicenter Retrospective Study Comparing 100 mg and 200 mg-IL PSO (Italian Landscape Psoriasis)
Abstract
Introduction: Tildrakizumab is a monoclonal antibody targeting interleukin (IL)-23 approved for the treatment of moderate-to-severe plaque psoriasis across two different dosages (100 mg and 200 mg). The higher dosage is recommended for patients with a body weight ≥ 90 kg or a high disease burden (Psoriasis Area and Severity Index [PASI] ≥ 16 or the involvement of difficult-to-treat areas). We conducted a 52-week multicenter retrospective study to compare the effectiveness and safety of both dosages and assess their impact on specific patient subgroups.
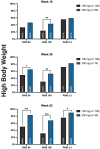
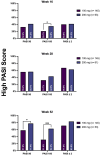
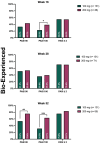
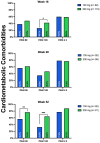
Methods: We enrolled a total of 540 patients with high disease burden or body weight ≥ 90 kg; 177 and 363 were treated with tildrakizumab 200 mg and 100 mg, respectively. The effectiveness was evaluated in terms of PASI 90, PASI 100, and PASI ≤ 2 at weeks 16, 28, and 52. We also performed subanalyses according to the body weight (≥ 90 kg), PASI ≥ 16, prior biologic exposure, involvement of difficult-to-treat areas, and the presence of at least one cardiometabolic comorbidity.
Results: After 16 weeks of treatment, a higher proportion of patients in the 200-mg group achieved PASI 90 and PASI 100 compared to those in the 100-mg group (43.5% vs. 34.3% and 36.4% vs. 24.2%, respectively). These results were sustained at 1 year, with PASI 90 and PASI 100 reached by 68.6% and 52.9% of patients in the 200-mg group, respectively, versus 57.3% and 35% in the 100-mg group. All subgroup analyses consistently indicated a trend toward greater effectiveness with tildrakizumab 200 mg, particularly in terms of PASI 90 and PASI 100 achievement at weeks 16 and 52. No differences in the safety profile were observed throughout the study period.
Conclusion: Our findings confirm the superior effectiveness of tildrakizumab 200 mg over 100 mg in specific subgroups of patients with a comparable safety profile across the study period.
Keywords: Anti-IL-23; Biologics; Psoriasis; Real-Life; Tildrakizumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Mario Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie, and Boehringer Ingelheim. Mario Valenti is an Editorial Board member of Dermatology and Therapy. Mario Valenti was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Luciano Ibba has been a consultant for Almirall. Luigi Gargiulo has been a consultant and/or speaker for Abbvie, Almirall, Eli Lilly, Pfizer, Sanofi and UCB Pharma. Piergiorgio Malagoli has been a speaker for AbbVie, Eli Lilly, Novartis, Janssen-Cilag, Celgene, Leo Pharma, and Almirall. Anna Balato has received honoraria for participation in advisory boards, meetings, or as a speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. Federico Bardazzi has been a consultant adviser and clinical study investigator for Eli Lilly, AbbVie, Novartis, Leo Pharma, Sandoz, Bristol Myers, Abiogen-Pharma, Celgene, and Janssen. Martina Burlando has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, and UCB Pharma. Carlo G. Carrera has served as a board participant or speaker for AbbVie, Eli Lilly, Janssen, Novartis, Celgene, Almirall, and Leo Pharma. Andrea Carugno has been a consultant and/or speaker for AbbVie, Leo Pharma, Eli Lilly, Novartis, Janssen-Cilag, Amgen, Almirall, UCB Pharma, and Boehringer Ingelheim. Paolo Dapavo has been a speaker for Novartis, AbbVie, Sanofi, UCB, Janssen, Eli Lilly, and Leo Pharma. Maria Concetta Fargnoli has served as a consultant/advisor, received speaker honoraria and/or grants, and/or is an investigator for Amgen, Almirall, AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Galderma, Kyowa Kirin, Incyte, Leo Pharma, Pierre Fabre, UCB, Eli Lilly, Pfizer, Janssen, MSD, Novartis, Sanofi, Regeneron, Sun Pharma, and Takeda. Francesca M. Gaiani acted as a speaker or consultant for Novartis, AbbVie, Eli Lilly, Celgene, Leo Pharma, and Almirall. Claudio Guarneri has been a scientific consultant, speaker, and clinical study investigator for AbbVie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Almirall, and Leo Pharma. Claudia Lasagni declares a conflict of interest with AbbVie, Novartis, Eli Lilly, and Almirall. Francesco Loconsole served on advisory boards and/or received honoraria for lectures from Abbvie, Janssen-Cilag, Novartis, Lilly, Sanofi. Angelo V. Marzano reports consultancy/advisory board disease-relevant honoraria from AbbVie, Boehringer Ingelheim, Novartis, Pfizer, Sanofi, and UCB. Matteo Megna acted as a speaker or consultant for AbbVie, Eli Lilly, Janssen, Leo Pharma, and Novartis. Diego Orsini has been a speaker and/or consultant for AbbVie, Leo Pharma, UCB, Bristol-Myers Squibb, and Boehringer Ingelheim. Simone Ribero has served as an advisory board member and/or consultant and has received fees and speaker’s honoraria or has participated in clinical studies for AbbVie, Almirall, Leo Pharma, Eli Lilly, Novartis, Pfizer, and Sanofi Genzyme. Davide Strippoli has been a consultant and/or speaker for AbbVie, Celgene, Janssen, Eli Lilly, Novartis, and Pfizer. Emanuele Trovato is involved in an intermittent project focused on consulting and/or advisory relationships and/or travel-congress support with Eli Lilly, Novartis, Janssen-Cilag, AbbVie, and Almirall. Marina Venturini served as a speaker or advisory board member for AbbVie, Almirall, Amgen, Eli Lilly, Galderma, Leo Pharma, Novartis, Pierre Fabre, and UCB Pharma. Leonardo Zichichi has received grants for scientific contributions from AbbVie, Almirall, Amgen, Eli Lilly, and Novartis. Antonio Costanzo has served as an advisory board member, consultant, and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, Leo Pharma, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB Pharma. Alessandra Narcisi has served on advisory boards, received honoraria for lectures, and research grants from Almirall, AbbVie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi Genzyme, Amgen, and Boehringer Ingelheim. Sara Di Giulio, Pina Brianti, Anna E. Cagni, Norma Cameli, Aldo Cuccia, Eugenia V. Di Brizzi, Valentina Dini, Gaetano Licata, Santo R. Mercuri, Alessandra Michelucci, Maria L. Muscumesi, Romina Ortega, Luca Potestio, Luca Rapparini, and Francesca Satolli have nothing to declare. Ethical Approval: Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received tildrakizumab as in good clinical practice, in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Figures


References
-
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–94. 10.1016/S0140-6736(14)61909-7. - PubMed
-
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60. 10.1001/jama.2020.4006. - PubMed
-
- European Medicines Agency. Ilumetri (tildrakizumab): summary of product characteristics. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/ilumetri. Accessed 2025 Feb 12.

