Varenicline for Youth Nicotine Vaping Cessation: A Randomized Clinical Trial
- PMID: 40266580
- PMCID: PMC12019676
- DOI: 10.1001/jama.2025.3810
Varenicline for Youth Nicotine Vaping Cessation: A Randomized Clinical Trial
Abstract
Importance: Electronic cigarette use (vaping) among adolescents and young adults is common. Few treatments have been tested in this population.
Objective: To evaluate the efficacy of varenicline for nicotine vaping cessation in youth who do not smoke tobacco regularly.
Design, setting, and participants: A 3-group randomized clinical trial compared 12 weeks of double-blind varenicline vs placebo, each added to brief, remotely delivered behavioral counseling and compared with single-blind enhanced usual care, with monthly follow-up to 24 weeks. The trial was conducted among youth, aged 16 to 25 years, who vaped nicotine daily or near daily, did not regularly smoke tobacco, and wanted to reduce or quit vaping, in a single US state from June 2022 to May 2024. Data collection ended May 28, 2024.
Interventions: Participants were randomized (1:1:1) to 12 weeks of varenicline titrated to 1 mg twice daily over 7 days (standard titration), weekly counseling, and referral to text messaging vaping cessation support (This is Quitting [TIQ]) (n = 88); identical placebo, weekly counseling, and referral to TIQ (n = 87); or enhanced usual care (referral to TIQ only) (n = 86).
Main outcomes and measures: Biochemically verified continuous vaping abstinence for the last 4 weeks of varenicline treatment vs placebo (primary outcome). Secondary outcomes included bioverified continuous abstinence from weeks 9 through 24 in the varenicline and placebo groups. Additional analyses compared varenicline group and placebo group with enhanced usual care.
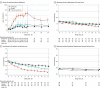
Results: Of 261 randomized participants (mean age, 21.4 years; 53% female), 254 completed the trial (97.3%). For varenicline and placebo, continuous abstinence rates were 51% vs 14% during weeks 9 through 12 (adjusted odds ratio [aOR], 6.5 [95% CI, 3.0-14.1]; P < .001) and 28% vs 7% during weeks 9 through 24 (aOR, 6.0 [95% CI, 2.1-16.9]; P < .001). Varenicline had higher continuous abstinence rates vs enhanced usual care during weeks 9 through 12 (51% vs 6%; aOR, 16.9 [95% CI, 6.2-46.3]) and during weeks 9 through 24 (28% vs 4%; aOR, 11.0 [95% CI, 3.1-38.8]). Continuous abstinence rates were not significantly different between the placebo and enhanced usual care groups. Study medication was generally well tolerated. Two varenicline participants (2%) and 1 placebo participant (1%) discontinued study medications due to adverse events. No drug-related serious adverse events occurred. Treatment-emergent adverse events were reported by 76 (86%) in the varenicline group, 68 (79%) in the placebo group, and 68 (79%) in the enhanced usual care group.
Conclusions and relevance: Varenicline, combined with behavioral counseling, increased vaping abstinence in youth who vape nicotine and do not regularly smoke tobacco.
Trial registration: ClinicalTrials.gov Identifier: NCT05367492.
Conflict of interest statement
Figures

References
-
- Center for Behavioral Health Statistics and Quality. Table 2.1B—tobacco product use, nicotine vaping, and alcohol use in lifetime, past year, and past month: among people aged 12 or older; by age group, percentages, 2022 and 2023. Substance Abuse and Mental Health Services Administration. Published 2024. Accessed March 11, 2025. https://www.samhsa.gov/data/sites/default/files/reports/rpt47100/NSDUHDe...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

