Macrophage-Hosted Porphyromonas gingivalis Is a Risk Factor for Cataract Development
- PMID: 40266593
- PMCID: PMC12025317
- DOI: 10.1167/iovs.66.4.68
Macrophage-Hosted Porphyromonas gingivalis Is a Risk Factor for Cataract Development
Abstract
Purpose: We studied the regulatory association of Porphyromonas gingivalis (PG) and cataracts.
Methods: PCR and FISH assays were used for detecting PG 16s ribosomal RNA genome, Immunofluorescence was for expression of RpgA in anterior capsular epithelium and fibrosis markers in anterior subcapsular cataract (ASC) model. Flow cytometry was for reactive oxygen species and apoptosis. RNA deep sequencing is for differential gene expression analysis.
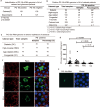
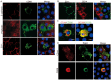
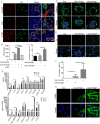
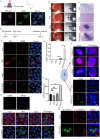
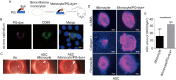
Results: PG's 16s ribosomal RNA gene is positively in 43.3% (101/233 cases) of aqueous humor (AH) samples of patients with cataracts, which differs from 4.7% (6/127) of PG-positive AH in patients with glaucoma. Diabetic and high myopia cataracts increase PG-positive AH compared with age-related cataracts. No PG is observed in AH of congenital cataracts. PG is positive in 82% to 94% of the cataractous anterior capsule tissues from high myopia and age-related, congenital, and diabetic cataracts. The PG-positive cells in the cataractous anterior capsular epithelium are CD68+/CD14+ macrophages, but not anterior epithelial cells. In rat ASC models, PG injected via the tail vein or PG-carried bone marrow monocytes can migrate into the equatorial lens epithelium in form of PG-positive macrophages, which promote ASC progression with upregulation of collagen, fibronectin and α smooth muscle actin (α-SMA) expression, and increase 8-OHdG levels and α-SMA expression in the surrounding lens epithelial cells. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analysis of the RNA sequencing dataset of ASC tissues shows that signaling pathways related to epithelial-mesenchymal transition, oxidative stress, and cell death are up-regulated in PG + ASC compared with that in ASC alone. Co-culture of supernatants of Raw264.7/PG+ cells with rat primary lens epithelial cells increases the 8-OHdG levels, mitochondrial fission, apoptosis, and expression of α-SMA.
Conclusions: Chronic infection with PG can access the lens epithelium via macrophages during stress conditions, which promotes cataract development by possibly elevating oxidative stress, apoptosis, and epithelial-mesenchymal transition in lens tissues. PG infection is a novel a risk factor for cataract development.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Arginase-1 promotes lens epithelial-to-mesenchymal transition in different models of anterior subcapsular cataract.Cell Commun Signal. 2023 Sep 18;21(1):236. doi: 10.1186/s12964-023-01210-4. Cell Commun Signal. 2023. PMID: 37723490 Free PMC article.
-
Slit2 Promotes H2O2-Induced Lens Epithelial Cells Oxidative Damage and Age-Related Cataract.Curr Eye Res. 2025 Jan;50(1):41-50. doi: 10.1080/02713683.2024.2388698. Epub 2024 Aug 14. Curr Eye Res. 2025. PMID: 39143744
-
Pigment epithelium-derived factor in cataractous aqueous humor and lens epithelial cells.Yan Ke Xue Bao. 2006 Mar;22(1):40-6, 53. Yan Ke Xue Bao. 2006. PMID: 17162928
-
[The oxidative stress in the cataract formation].Nippon Ganka Gakkai Zasshi. 1995 Dec;99(12):1303-41. Nippon Ganka Gakkai Zasshi. 1995. PMID: 8571853 Review. Japanese.
-
Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation.Cells Tissues Organs. 2005;179(1-2):43-55. doi: 10.1159/000084508. Cells Tissues Organs. 2005. PMID: 15942192 Review.
References
-
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S.. Age-related cataract. Lancet. 2005; 365: 599–609. - PubMed
-
- Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS.. Cataracts. Lancet. 2017; 390: 600–612. - PubMed
-
- Ang MJ, Afshari NA.. Cataract and systemic disease: a review. Clin Exp Ophthalmol. 2021; 49: 118–127. - PubMed
-
- York A. P. gingivalis drives carcinoma progression. Nat Rev Microbiol. 2020; 18: 674. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

