Rahnella aquatilis Isolated from Aedes albopictus Impairs Mosquito Reproduction Capacity
- PMID: 40266721
- PMCID: PMC11942639
- DOI: 10.3390/insects16030257
Rahnella aquatilis Isolated from Aedes albopictus Impairs Mosquito Reproduction Capacity
Abstract
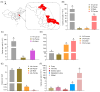
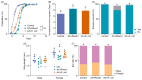
Aedes albopictus is one of the most important vectors of Dengue, which poses a serious threat to public health. The bacterial microbiota has an effect on the parameters of mosquitos, such as larval development and fecundity, and it has emerged as a promising field to be explored for novel environmentally friendly control strategies. Rahnella sp. are present in many insects, including Ae. Albopictus, and play a role in bacterial-insect interactions; however, the role of the bacteria in mosquito biology has not yet been characterized. In this study, we characterized the Rahnella isolate RAeA1 obtained from Ae. albopcitus, and its colonization stability in Ae. albopictus was investigated by generating GFP-tagged bacteria. The influences of the bacteria on larval development and mosquito reproductive capacity were evaluated by inoculating RAeA1 in axenic larvae and antibiotic-treated adult mosquitoes, respectively. The results indicated that RAeA1, which is widespread in the field population of Ae. albopictus, can be transmitted directly from the parental strain to the progeny and can rescue axenic larvae developing into adults with a prolonged development time to pupation. RAeA1 inoculation can impair egg production and ovary maturation, as well as reducing the synthesis of ecdysteroids and vitellogenin in Ae. albopictus females. Overall, our results provide a thorough study of bacterium function characterization that will facilitate the development of potential strategies in relation to the design of microbiomes for vector control.
Keywords: Aedes albopictus; Rahnella aquatilis; microbiota; mosquito reproduction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Impact of deltamethrin-resistance in Aedes albopictus on its fitness cost and vector competence.PLoS Negl Trop Dis. 2021 Apr 27;15(4):e0009391. doi: 10.1371/journal.pntd.0009391. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33905415 Free PMC article.
-
Mosquito densovirus significantly reduces the vector susceptibility to dengue virus serotype 2 in Aedes albopictus mosquitoes (Diptera: Culicidae).Infect Dis Poverty. 2023 May 9;12(1):48. doi: 10.1186/s40249-023-01099-8. Infect Dis Poverty. 2023. PMID: 37161462 Free PMC article.
-
Diversity of midgut bacteria in larvae and females of Aedes aegypti and Aedes albopictus from Gampaha District, Sri Lanka.Parasit Vectors. 2021 Aug 28;14(1):433. doi: 10.1186/s13071-021-04900-5. Parasit Vectors. 2021. PMID: 34454583 Free PMC article.
-
Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species.Insect Sci. 2016 Apr;23(2):162-74. doi: 10.1111/1744-7917.12291. Epub 2016 Jan 18. Insect Sci. 2016. PMID: 26542083 Review.
-
Critical review of the vector status of Aedes albopictus.Med Vet Entomol. 2004 Sep;18(3):215-27. doi: 10.1111/j.0269-283X.2004.00513.x. Med Vet Entomol. 2004. PMID: 15347388 Review.
References
-
- Battaglia V., Agostini V., Moroni E., Colombo G., Lombardo G., Rambaldi Migliore N., Gabrieli P., Garofalo M., Gagliardi S., Gomulski L.M., et al. The worldwide spread of Aedes albopictus: New insights from mitogenomes. Front. Genet. 2022;13:931163. doi: 10.3389/fgene.2022.931163. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

