Assessment of the Osseointegration of Pure-Phase β-Tricalcium Phosphate (β-TCP) Ceramic Cylinder Implants in Critical Segmental Radial Bone Defects in Rabbits
- PMID: 40266960
- PMCID: PMC11946808
- DOI: 10.3390/vetsci12030200
Assessment of the Osseointegration of Pure-Phase β-Tricalcium Phosphate (β-TCP) Ceramic Cylinder Implants in Critical Segmental Radial Bone Defects in Rabbits
Abstract
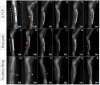
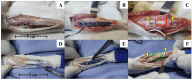
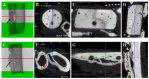
Autografts, allografts, and synthetic bone substitutes are essential in reconstructive orthopedic surgery. Although autografts and allografts provide excellent skeletal integration, their use is limited by host morbidity and graft acquisition challenges. Synthetic materials like β-tricalcium phosphate (β-TCP) offer promising osseoconductive properties as a potential substitute. This study evaluated the osseointegration of β-TCP ceramic cylinder implants in bone defects in rabbits. Eighteen New Zealand rabbits underwent radial diaphysis ostectomy to create a critical segmental defect and were divided into three groups: Group A received β-TCP blocks, Group B received allogenous cortical bone grafts, and Group C underwent ostectomy without defect filling. Postoperative assessments included clinical evaluations, radiographs, micro-computed tomography, and histology at various time points to assess osseointegration and implant resorption. At the 120th postoperative day, Group B showed successful bone integration without infection. In contrast, Group A showed no osseointegration or resorption of the β-TCP implants, and Group C exhibited bone non-union. While β-TCP demonstrated biocompatibility, it lacked osseoconductivity, likely due to low porosity. β-TCP implants did not promote bone consolidation, suggesting that further research on porosity and shape is needed to improve their suitability for veterinary orthopedic reconstructive surgery.
Keywords: biomaterial; bone critical defect; bone implant; bone segmental defect.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Evaluation of Osseointegration and Bone Healing Using Pure-Phase β - TCP Ceramic Implant in Bone Critical Defects. A Systematic Review.Front Vet Sci. 2022 Jul 12;9:859920. doi: 10.3389/fvets.2022.859920. eCollection 2022. Front Vet Sci. 2022. PMID: 35909673 Free PMC article.
-
Repair of goat tibial defects with bone marrow stromal cells and beta-tricalcium phosphate.J Mater Sci Mater Med. 2008 Jun;19(6):2367-76. doi: 10.1007/s10856-007-3348-3. Epub 2007 Dec 25. J Mater Sci Mater Med. 2008. PMID: 18158615
-
Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery.Eur Spine J. 2001 Oct;10 Suppl 2(Suppl 2):S132-40. doi: 10.1007/s005860100325. Eur Spine J. 2001. PMID: 11716010 Free PMC article.
-
Use of a three-dimensional printed polylactide-coglycolide/tricalcium phosphate composite scaffold incorporating magnesium powder to enhance bone defect repair in rabbits.J Orthop Translat. 2018 Aug 16;16:62-70. doi: 10.1016/j.jot.2018.07.007. eCollection 2019 Jan. J Orthop Translat. 2018. PMID: 30723682 Free PMC article.
-
Adjuvant therapies of bone graft around non-cemented experimental orthopedic implants stereological methods and experiments in dogs.Acta Orthop Suppl. 2008 Aug;79(330):1-43. Acta Orthop Suppl. 2008. PMID: 19065776 Review.
References
-
- Dasgupta S., Maji K., Nandi S.K. Investigating the mechanical, physiochemical and osteogenic properties in gelatin-chitosan-bioactive nanoceramic composite scaffolds for bone tissue regeneration: In vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;94:713–728. doi: 10.1016/j.msec.2018.10.022. - DOI - PubMed
-
- Sharma A.K., Gupta S. Microwave processing of biomaterials for orthopedic implants: Challenges and possibilities. JOM. 2020;72:1211–1228. doi: 10.1007/s11837-020-04003-z. - DOI
LinkOut - more resources
Full Text Sources

