A selfish supergene causes meiotic drive through both sexes in Drosophila
- PMID: 40267129
- PMCID: PMC12054836
- DOI: 10.1073/pnas.2421185122
A selfish supergene causes meiotic drive through both sexes in Drosophila
Abstract
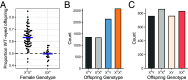
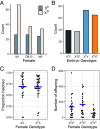
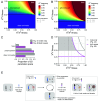
Meiotic drivers are selfish genetic elements that bias their own transmission during meiosis or gamete formation. Due to the fundamental differences between male and female meiosis in animals and plants, meiotic drivers operate through distinct mechanisms in the two sexes: In females, they exploit the asymmetry of meiosis to ensure their inclusion in the egg, whereas in males, they eliminate competing gametes after symmetric meiosis. Meiotic drive is commonly reported in males, where it strongly influences the evolution of spermatogenesis, while the few known cases in females have highlighted its crucial role in centromere evolution. Despite a growing number of examples in a wide range of organisms, meiotic drive has so far only been observed in one sex or the other since its discovery nearly 100 y ago. Here, we show that a selfish X chromosome known to cause meiotic drive in male Drosophila testacea flies also causes meiotic drive in females. We find that this X chromosome has supergene architecture, harboring extensive structural rearrangements that suppress recombination between the two X chromosomes. This has contributed to a substantial expansion of its size compared to the wild-type chromosome, partly due to the accumulation of species-specific repetitive elements. Our findings suggest that female meiotic drive may play an important role in the evolutionary dynamics of polymorphic structural variants that suppress recombination, including inversions, translocations, and supergenes.
Keywords: meiotic drive; selfish genetic elements; sex chromosome; supergenes.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

