Conformational plasticity of mitochondrial VDAC2 controls the kinetics of its interaction with cytosolic proteins
- PMID: 40267181
- PMCID: PMC12017312
- DOI: 10.1126/sciadv.adv4410
Conformational plasticity of mitochondrial VDAC2 controls the kinetics of its interaction with cytosolic proteins
Abstract
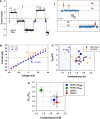
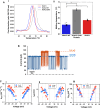
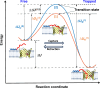
The voltage-dependent anion channel (VDAC) is a key conduit of the mitochondrial outer membrane for water-soluble metabolites and ions. Among the three mammalian isoforms, VDAC2 is unique because of its embryonic lethality upon knockout. Using single-molecule electrophysiology, we investigate the biophysical properties that distinguish VDAC2 from VDAC1 and VDAC3. Unlike the latter, VDAC2 exhibits dynamic switching between multiple high-conductance, anion-selective substates. Using α-synuclein (αSyn)-a known VDAC1 cytosolic regulator-we found that higher-conductance substates correlate with increased on-rates of αSyn-VDAC2 interaction but shorter blockage times, maintaining a consistent equilibrium constant across all substates. This suggests that αSyn detects VDAC2's dynamic structural variations before final binding. We explored the dependence of VDAC2's unique amino-terminal extension and cysteines on substate behavior, finding that both structural elements modulate substate occurrence. The discovered conformational flexibility enables VDAC2 recognition by diverse binding partners, explaining its critical physiological role via dynamical adaptation to mitochondrial metabolic conditions.
Figures




References
-
- Rahmani Z., Maunoury C., Siddiqui A., Isolation of a novel human voltage-dependent anion channel gene. Eur. J. Hum. Genet. 6, 337–340 (1998). - PubMed
-
- Messina A., Reina S., Guarino F., De Pinto V., VDAC isoforms in mammals. Biochim. Biophys. Acta 1818, 1466–1476 (2012). - PubMed
-
- Piehowski P. D., Zhu Y., Bramer L. M., Stratton K. G., Zhao R., Orton D. J., Moore R. J., Yuan J., Mitchell H. D., Gao Y., Webb-Robertson B. M., Dey S. K., Kelly R. T., Burnum-Johnson K. E., Automated mass spectrometry imaging of over 2000 proteins from tissue sections at 100-μm spatial resolution. Nat. Commun. 11, 8 (2020). - PMC - PubMed
-
- Sampson M. J., Decker W. K., Beaudet A. L., Ruitenbeek W., Armstrong D., Hicks M. J., Craigen W. J., Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 276, 39206–39212 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

