Synaptophysin accelerates synaptic vesicle fusion by expanding the membrane upon neurotransmitter loading
- PMID: 40267188
- PMCID: PMC12017324
- DOI: 10.1126/sciadv.ads4661
Synaptophysin accelerates synaptic vesicle fusion by expanding the membrane upon neurotransmitter loading
Abstract
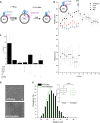
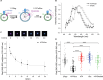
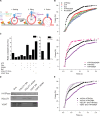
Synaptic transmission is mediated by the exocytotic release of neurotransmitters stored in synaptic vesicles (SVs). SVs filled with neurotransmitters preferentially undergo exocytosis, but it is unclear how this is achieved. Here, we show that during transmitter loading, SVs substantially increase in size, which is reversible and requires synaptophysin, an abundant membrane protein with an unclear function. SVs are larger when synaptophysin is knocked out, and conversely, liposomes are smaller when reconstituted with synaptophysin. Moreover, transmitter loading of SVs accelerates fusion in vitro, which is abolished when synaptophysin is lacking despite near normal transmitter uptake. We conclude that synaptophysin functions as a curvature-promoting entity in the SV membrane, allowing for major lateral expansion of the SV membrane during neurotransmitter filling, thus increasing their propensity for exocytosis.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

