Structural dissection of ergosterol metabolism reveals a pathway optimized for membrane phase separation
- PMID: 40267201
- PMCID: PMC12017304
- DOI: 10.1126/sciadv.adu7190
Structural dissection of ergosterol metabolism reveals a pathway optimized for membrane phase separation
Abstract
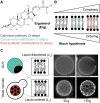
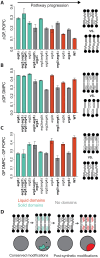
Sterols are among the most abundant lipids in eukaryotic cells yet are synthesized through notoriously long metabolic pathways. It has been proposed that the molecular evolution of such pathways must have required each step to increase the capacity of its product to condense and order phospholipids. Here, we carry out a systematic analysis of the ergosterol pathway that leverages the yeast vacuole's capacity to phase separate into ordered membrane domains. In the post-synthetic steps specific to ergosterol biosynthesis, we find that successive modifications act to oscillate ordering capacity, settling on a level that supports phase separation while retaining fluidity of the resulting domains. Simulations carried out with each intermediate showed how conformers in the sterol's alkyl tail are capable of modulating long-range ordering of phospholipids, which could underlie changes in phase behavior. Our results indicate that the complexity of sterol metabolism could have resulted from the need to balance lipid interactions required for membrane organization.
Figures



Update of
-
Dissection of ergosterol metabolism reveals a pathway optimized for membrane phase separation.bioRxiv [Preprint]. 2025 Jan 5:2025.01.05.631358. doi: 10.1101/2025.01.05.631358. bioRxiv. 2025. Update in: Sci Adv. 2025 Apr 25;11(17):eadu7190. doi: 10.1126/sciadv.adu7190. PMID: 40568137 Free PMC article. Updated. Preprint.
References
-
- Brocks J. J., Nettersheim B. J., Adam P., Schaeffer P., Jarrett A. J. M., Güneli N., Liyanage T., van Maldegem L. M., Hallmann C., Hope J. M., Lost world of complex life and the late rise of the eukaryotic crown. Nature 618, 767–773 (2023). - PubMed
-
- W. R. Nes, W. D. Nes, “Phylogenetics and biosynthesis” in Lipids in Evolution, W. R. Nes, W. D. Nes, Eds. (Springer, 1980), pp. 157–192.
-
- K. Bloch, Blondes in Venetian Paintings, the Nine-Banded Armadillo, and Other Essays in Biochemistry (Yale Univ. Press, 1994).

