A high-resolution screen identifies a preexisting beta-lactam that specifically treats Lyme disease in mice
- PMID: 40267215
- PMCID: PMC12258496
- DOI: 10.1126/scitranslmed.adr9091
A high-resolution screen identifies a preexisting beta-lactam that specifically treats Lyme disease in mice
Abstract
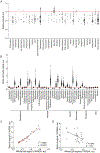
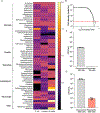
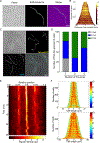
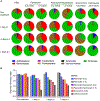
Lyme disease, caused by Borrelia burgdorferi in the United States, is an escalating human health problem that can cause severe disease if not properly treated. Doxycycline is the primary treatment option for Lyme disease; however, several concerns are associated with high-dose doxycycline treatment. For example, doxycycline is a broad-spectrum antibiotic and kills beneficial bacteria. Doxycycline is also known to produce unwanted off-target effects in eukaryotic cells. Some at-risk populations such as young children cannot be prescribed doxycycline, and in addition to these shortcomings, the treatment appears to fail in 10 to 20% of cases. We reasoned that safe, alternative therapies may currently exist but have not yet been found because of the challenges associated with drug screening approaches. We screened nearly 500 US Food and Drug Administration-approved compounds using an array of physiological, cellular, and molecular techniques. Top-performing candidates were counter screened to identify compounds that did not affect other bacterial phyla. Piperacillin emerged as a compound that eradicated B. burgdorferi at low-nanomolar concentrations by specifically interfering with the unusual, multizonal peptidoglycan synthesis pattern common to the Borrelia clade. Mechanistic in vitro studies identified the cellular target of piperacillin in B. burgdorferi and produced key insights that may explain both the specificity and efficacy of the compound. Further, in vivo studies using an experimental mouse infection model demonstrated that piperacillin treated animals at a 100-fold lower dose than the effective dose of doxycycline without affecting the murine microbiome. Our findings suggest that piperacillin may offer clinicians another therapeutic option for Lyme disease.
Figures

References
-
- Bobe JR, Jutras BL, Horn EJ, Embers ME, Bailey A, Moritz RL, Zhang Y, Soloski MJ, Ostfeld RS, Marconi RT, Aucott J, Ma’ayan A, Keesing F, Lewis K, Ben Mamoun C, Rebman AW, McClune ME, Breitschwerdt EB, Reddy PJ, Maggi R, Yang F, Nemser B, Ozcan A, Garner O, Di Carlo D, Ballard Z, Joung HA, Garcia-Romeu A, Griffiths RR, Baumgarth N, Fallon BA, Recent progress in Lyme disease and remaining challenges. Front. Med 8, 666554 (2021). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

