Development of a core outcome set for clinical trials targeting interventions aiming to improve adherence to appropriate polypharmacy in older people-an international consensus study
- PMID: 40267306
- PMCID: PMC12017393
- DOI: 10.1093/ageing/afaf102
Development of a core outcome set for clinical trials targeting interventions aiming to improve adherence to appropriate polypharmacy in older people-an international consensus study
Abstract
Background: Medication non-adherence is prevalent in older people taking polypharmacy. Several interventions have been employed to improve adherence in this population. However, inconsistencies in outcomes have impeded comparisons of findings. Accordingly, this work aimed to develop a core outcome set (COS) for use in trials aiming to improve adherence to appropriate polypharmacy in older people.
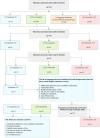
Methods: A group of stakeholders, including academics, journal editors, healthcare professionals (HCPs) and public participants, evaluated 13 outcomes compiled from the literature in a Delphi study using a nine-point Likert scale ranging from 1 to 9, where higher scores (7-9) indicated critical importance and lower scores (1-3) unimportance. The resultant Delphi consensus list was discussed and voted on (yes: critical and no: unimportant) in two online nominal group technique (NGT) meetings. The NGT followed a five-stage approach: introduction, silent generation, round-robin, clarification and voting. An outcome was included if ≥80% of participants scored it critical and ≤ 15% scored it as unimportant.
Results: Of the 13 outcomes originally presented to participants, consensus was achieved to include six outcomes in the COS after the Delphi study (Round 1, n = 57; Round 2, n = 53; Round 3, n = 50, where 'n' represents participant numbers) and the NGT meetings (n = 10) comprising medication adherence across multiple medications, treatment burden, health-related quality of life (HRQoL), healthcare utilisation (HCU), adverse events and side effects (AEs and SEs) and cost-effectiveness.
Conclusion: This COS should be used in intervention studies focusing on improving adherence to appropriate polypharmacy in older people. Future work should identify outcome measurement instruments to be used alongside the COS.
Keywords: adherence; core outcome set; older people; outcomes; polypharmacy.
© The Author(s) 2025. Published by Oxford University Press on behalf of the British Geriatrics Society.
Conflict of interest statement
None.
Figures
Similar articles
-
Core Outcome Set for Trials Aimed at Improving the Appropriateness of Polypharmacy in Older People in Primary Care.J Am Geriatr Soc. 2018 Jul;66(6):1206-1212. doi: 10.1111/jgs.15245. Epub 2018 Feb 20. J Am Geriatr Soc. 2018. PMID: 29461621
-
Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes.Trials. 2017 Apr 12;18(1):175. doi: 10.1186/s13063-017-1915-6. Trials. 2017. PMID: 28403876 Free PMC article.
-
International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy.BMC Med. 2018 Feb 13;16(1):21. doi: 10.1186/s12916-018-1007-9. BMC Med. 2018. PMID: 29433501 Free PMC article.
-
Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications.Cochrane Database Syst Rev. 2020 May 8;5(5):CD012419. doi: 10.1002/14651858.CD012419.pub2. Cochrane Database Syst Rev. 2020. PMID: 32383493 Free PMC article.
-
A core outcomes set for clinical trials of interventions for young adults with type 1 diabetes: an international, multi-perspective Delphi consensus study.Trials. 2017 Dec 19;18(1):602. doi: 10.1186/s13063-017-2364-y. Trials. 2017. PMID: 29258565 Free PMC article.
References
-
- Wilmoth JR, Bas D, Mukherjee S et al. World Social Report 2023. New York, NY: United Nations Department of Economic and Social Affairs, 2023.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical