Recent Advances in Augmenting the Therapeutic Efficacy of Peptide-Drug Conjugates
- PMID: 40267310
- PMCID: PMC12067445
- DOI: 10.1021/acs.jmedchem.5c00007
Recent Advances in Augmenting the Therapeutic Efficacy of Peptide-Drug Conjugates
Abstract
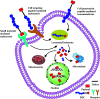
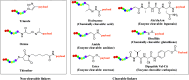
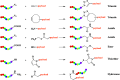
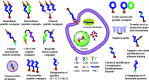
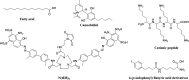
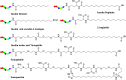
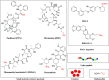
There is an urgent need for the development of safe and effective modalities for the treatment of diseases owing to drug resistance, undesired side effects, and poor clinical outcomes. Combining cell-targeting and efficient cell-killing properties, peptide-drug conjugates (PDCs) have demonstrated superior efficacy compared with peptides and payloads alone. However, innovative molecular designs of PDCs are essential for further improving targeting precision, protease resistance and stability, cell permeability, and overall treatment efficacy. Several strategies have been developed to address these challenges, such as multivalency approaches, bispecific targeting, and long-acting PDCs. Other novel strategies, including overcoming biological barriers, conjugating novel functional payloads, and targeting macropinocytosis, have also shown promise. This perspective compiles the most recent strategies for enhancing PDC treatment efficacy, highlights key advancements in PDC, and provides insights on future directions for the development of novel PDCs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

