Angiotensin II-induced cardiac fibrosis and dysfunction are exacerbated by deletion of cGKI in periostin+ myofibroblasts
- PMID: 40267335
- PMCID: PMC12247847
- DOI: 10.1042/CS20241204
Angiotensin II-induced cardiac fibrosis and dysfunction are exacerbated by deletion of cGKI in periostin+ myofibroblasts
Abstract
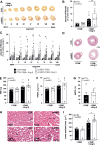
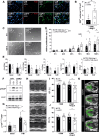
Differentiation of cardiac fibroblasts (CF) into myofibroblasts (CMFs) is considered a critical event in response to the maladaptive cardiac remodeling triggered by angiotensin II (Ang II). Active CMFs are proliferative and contribute to the production of extracellular matrix and matricellular proteins such as periostin, to myocardial fibrosis and thus muscle stiffness. Although previous studies provided substantial evidence for the antifibrotic signaling elicited by NO/NP-cGMP-cGKI, the role of this axis in modulating CMF function(s) in vivo remains unclear.To address this, Ang II was delivered through osmotic minipumps into tamoxifen-induced CMF-specific cGKI knockout (cmfKO) and littermate control (CTR) male mice. CMF-restricted Cre activity in periostin+ cells resulted in an effective depletion of the cGKI protein observed in myocardial sections and in primary CF/CMF protein lysates obtained from Ang II-and tamoxifen-treated cmfKO. Although both genotypes responded identically to Ang II in terms of blood pressure and cardiac enlargement, cmfKO hearts showed significantly increased cardiomyocyte cross-sectional areas and developed a marked increase in myocardial fibrosis. Moreover, non-invasive echocardiography revealed a structure-related distortion of global systolic function and longitudinal deformation capacity in cmfKO versus CTR. Consistent with the results obtained in vivo, we observed a higher proliferation rate of CF/CMF derived from Ang II-treated cmfKO hearts compared to respective CTR cells as well as an increase in cardiomyocyte apoptosis in the absence of cGKI in periostin+ CMF. Our data confirm that endogenous cGKI function in periostin+ CMFs counteracts the Ang II-induced morphologic and structural changes that impair cardiomyocyte survival ultimately causing loss of heart function in male mice.
Keywords: angiotensin II; cGKI; cGMP; cardiac myofibroblasts; cardiac remodeling; fibrosis; periostin.
© 2025 The Author(s).
Conflict of interest statement
All other authors have explicitly declared that there are no conflicts of interest related to this article.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

