Integrating endocannabinoid signaling, CCK interneurons, and hippocampal circuit dynamics in behaving animals
- PMID: 40267911
- PMCID: PMC12410895
- DOI: 10.1016/j.neuron.2025.03.016
Integrating endocannabinoid signaling, CCK interneurons, and hippocampal circuit dynamics in behaving animals
Abstract
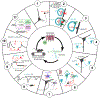
The brain's endocannabinoid signaling system modulates a diverse range of physiological phenomena and is also involved in various psychiatric and neurological disorders. The basic components of the molecular machinery underlying endocannabinoid-mediated synaptic signaling have been known for decades. However, limitations associated with the short-lived nature of endocannabinoid lipid signals had made it challenging to determine the spatiotemporal specificity and dynamics of endocannabinoid signaling in vivo. Here, we discuss how novel technologies have recently enabled unprecedented insights into endocannabinoid signaling taking place at specific synapses in behaving animals. In this review, we primarily focus on cannabinoid-sensitive inhibition in the hippocampus in relation to place cell properties to illustrate the potential of these novel methodologies. In addition, we highlight implications of these approaches and insights for the unraveling of cannabinoid regulation of synapses in vivo in other brain circuits in both health and disease.
Keywords: CB1 receptor; behavior; brain state; cholecystokinin; disease; endocannabinoid; hippocampus; inhibition; interneuron; translational.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



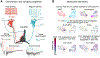
Similar articles
-
Cannabinoid Modulation of Excitability and Short-Term Neuronal Dynamics in the Dorsal and Ventral Hippocampus.Biology (Basel). 2025 May 31;14(6):642. doi: 10.3390/biology14060642. Biology (Basel). 2025. PMID: 40563893 Free PMC article.
-
Modulation of Neurexins Alternative Splicing by Cannabinoid Receptors 1 (CB1) Signaling.Cells. 2025 Jun 25;14(13):972. doi: 10.3390/cells14130972. Cells. 2025. PMID: 40643493 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review.Br J Pharmacol. 2015 Feb;172(3):737-53. doi: 10.1111/bph.12944. Br J Pharmacol. 2015. PMID: 25257544 Free PMC article.
References
-
- Maccarrone M, Di Marzo V, Gertsch J, Grether U, Howlett AC, Hua T, Makriyannis A, Piomelli D, Ueda N, and van der Stelt M (2023). Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev 75, 885–958. 10.1124/pharmrev.122.000600. - DOI - PMC - PubMed
-
- Herrlinger SA, Rao BY, Paredes MEC, Tuttman AL, Arain H, Varol E, Gogos JA, and Losonczy A (2024). Disorganized Inhibitory Dynamics and Functional Connectivity in Hippocampal area CA1 of 22q11.2 Deletion Mutant Mice. Preprint at bioRxiv. 10.1101/2024.04.28.591464. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

