CPT1C deficiency in SF1 neurons impairs early metabolic adaptation to dietary fats, leading to obesity
- PMID: 40268191
- PMCID: PMC12076790
- DOI: 10.1016/j.molmet.2025.102155
CPT1C deficiency in SF1 neurons impairs early metabolic adaptation to dietary fats, leading to obesity
Abstract
Objectives: SF1 neurons of the ventromedial hypothalamus (VMH) play a pivotal role in regulating body weight and adiposity, particularly in response to a high-fat diet (HFD), as well as in the recovery from insulin-induced hypoglycemia. While the brain-specific CPT1C isoform is well known for its role in controlling food intake and energy homeostasis, its function within specific hypothalamic neuronal populations remains largely unexplored. Here, we explore the role of CPT1C in SF1 neurons.
Methods: Mice deficient in CPT1C within SF1 neurons were generated, and their response to a HFD was investigated.
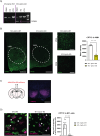
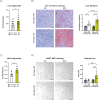
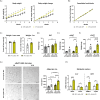
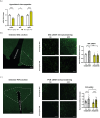
Results: SF1-Cpt1c-KO mice fail to adjust their caloric intake during initial HFD exposure, which is associated with impaired activation of the melanocortin system. Furthermore, these mice exhibit disrupted metabolic gene expression in the liver, muscle, and adipose tissue, leading to increased adiposity independently of food intake. In contrast, their response to glucose or insulin challenges remains intact. After long-term HFD exposure, SF1-Cpt1c-KO mice are more prone to developing obesity and glucose intolerance than control littermates, with males exhibiting a more severe phenotype. Interestingly, CPT1C deficiency in SF1 neurons also results in elevated hypothalamic endocannabinoid (eCB) levels under both chow and HFD conditions. We propose that this sustained eCB elevation reduces VMH activation by fatty acids and impairs the SF1-POMC drive upon fat intake.
Conclusion: Our findings establish CPT1C in SF1 neurons as essential for VMH-driven dietary fat sensing, satiety, and lipid metabolic adaptation.
Keywords: Adiposity; CPT1C; Endocannabinoids; Food intake; High-fat diet; SF1 neurons.
Copyright © 2025 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
CPT1C in the ventromedial nucleus of the hypothalamus is necessary for brown fat thermogenesis activation in obesity.Mol Metab. 2019 Jan;19:75-85. doi: 10.1016/j.molmet.2018.10.010. Epub 2018 Nov 2. Mol Metab. 2019. PMID: 30448371 Free PMC article.
-
Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake.Diabetologia. 2009 May;52(5):912-20. doi: 10.1007/s00125-009-1284-0. Epub 2009 Feb 18. Diabetologia. 2009. PMID: 19224198
-
Loss of CREB Coactivator CRTC1 in SF1 Cells Leads to Hyperphagia and Obesity by High-fat Diet But Not Normal Chow Diet.Endocrinology. 2021 Sep 1;162(9):bqab076. doi: 10.1210/endocr/bqab076. Endocrinology. 2021. PMID: 33846709 Free PMC article.
-
New Insights of SF1 Neurons in Hypothalamic Regulation of Obesity and Diabetes.Int J Mol Sci. 2021 Jun 8;22(12):6186. doi: 10.3390/ijms22126186. Int J Mol Sci. 2021. PMID: 34201257 Free PMC article. Review.
-
Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity.FEBS J. 2011 Feb;278(4):552-8. doi: 10.1111/j.1742-4658.2010.07978.x. Epub 2010 Dec 30. FEBS J. 2011. PMID: 21199367 Review.
Cited by
-
Beta-hydroxybutyrate counteracts the deleterious effects of a saturated high-fat diet on synaptic AMPAR receptors and cognitive performance.Mol Metab. 2025 Sep;99:102207. doi: 10.1016/j.molmet.2025.102207. Epub 2025 Jul 6. Mol Metab. 2025. PMID: 40628351 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

