Cost-effectiveness analysis of pembrolizumab plus chemotherapy as first-line treatment for advanced biliary tract cancer: perspectives from US and Chinese payers
- PMID: 40268484
- PMCID: PMC12020752
- DOI: 10.1136/bmjopen-2024-094047
Cost-effectiveness analysis of pembrolizumab plus chemotherapy as first-line treatment for advanced biliary tract cancer: perspectives from US and Chinese payers
Abstract
Background: The KEYNOTE-966 study demonstrated that pembrolizumab combined with chemotherapy is more effective than chemotherapy alone as first-line treatment for patients with advanced biliary tract cancer (BTC). However, the cost-effectiveness of pembrolizumab combined with chemotherapy in the USA and China remains uncertain.
Objective: This study aimed to evaluate the cost-effectiveness of pembrolizumab plus chemotherapy compared with placebo plus chemotherapy from the perspective of US and Chinese payers.
Design: Markov models with three health states were developed to simulate the process of advanced BTC. Cost data were obtained from available databases and published literature in the US scenario, and from local institutions from the China scenario. Utility values were derived from previous studies.
Outcome measures: Primary outcomes included quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs).
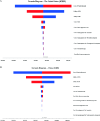
Results: In the US scenario, pembrolizumab plus chemotherapy increased costs by US$97,222.13, compared with chemotherapy alone, with a gain of 0.12 QALYs, resulting in an ICER of US$810 184.42 per QALY. In the China scenario, the ICER was $360 933.50 per QALY. Sensitivity analyses indicated the costs of pembrolizumab had the greatest impact on the model in both scenarios. Further analyses suggested that the optimal price of pembrolizumab in the USA would be nearly US$10.33 /mg, while a price reduction of over 90% would be required for the combined therapy to be cost-effective for patients in China.
Conclusion: Based on the willingness-to-pay threshold set at three times the gross domestic product per capita, pembrolizumab plus chemotherapy is not a cost-effective option for patients with advanced BTC in either the USA or China. Significant price reduction for pembrolizumab may be necessary to achieve an acceptable ICER.
Trial registration number: NCT04003636; postresults.
Keywords: HEALTH ECONOMICS; Hepatobiliary tumours; IMMUNOLOGY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures


References
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
