Ad-E6/7-HR vaccine improves the prophylactic and therapeutic efficacy in HPV-associated cancers
- PMID: 40268519
- PMCID: PMC12017896
- DOI: 10.1002/ctm2.70305
Ad-E6/7-HR vaccine improves the prophylactic and therapeutic efficacy in HPV-associated cancers
Abstract
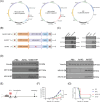
Background: High-risk human papillomavirus (HPV), especially HPV16, is closely correlated with certain cancers. E6 and E7 proteins of HPV16 play critical roles in oncogenesis, making them optimal targets for treating HPV-associated cancers. Here, we engineered an innovative vaccine, Ad-E6/7-HR, designed to evoke immune responses through the incorporation of self-assembling heptad-repeat 1 (HR1) and HR2 originated from Severe acute respiratory syndrome coronavirus 2.
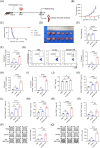
Methods: Ad-E6/7-HR was constructed utilising a replication-defective human adenovirus serotype 5 vector and evaluated its immunogenicity and therapeutic efficacy in murine models. We verified the antitumour efficacy of the vaccine in TC-1 subcutaneous and pulmonary models. Flow cytometry, enzyme-linked immunospot assay, and immunofluorescence staining were used to assess the cellular immunogenicity of Ad-E6/7-HR.
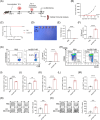
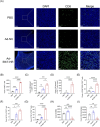
Results: Ad-E6/7-HR induced robust immune responses, significantly increasing antigen-specific CD8+ T cells. The vaccine also enhanced memory T-cell generation and induced potent cytokine secretion, as exemplified by interferon-γ and tumour necrosis factor-α. Ad-E6/7-HR conferred complete protection against tumour growth in the prophylactic model. In therapeutic settings, Ad-E6/7-HR significantly reduced tumour size and improved survival. Furthermore, Ad-E6/7-HR reshaped the tumour microenvironment by increased CD8+ T-cell recruitment and reduced immunosuppressive cells, like myeloid-derived suppressor cells and M2 macrophages, thereby enhancing antitumour immunity.
Conclusions: By targeting HPV16 E6 and E7 proteins and leveraging the self-assembling HR1 and HR2 sequences to enhance immune responses, Ad-E6/7-HR represented a promising candidate for preventing and treating HPV-associated cancers. Further clinical investigation is warranted to evaluate its potential in human trials.
Keywords: adenovirus vaccine; cellular immunity; human‐papillomavirus‐associated cancers; oncology.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
