Stress-induced phase separation in plastics drives the release of amorphous polymer micropollutants into water
- PMID: 40268905
- PMCID: PMC12018937
- DOI: 10.1038/s41467-025-58898-w
Stress-induced phase separation in plastics drives the release of amorphous polymer micropollutants into water
Abstract
Residual stress is an intrinsic property of semicrystalline plastics such as polypropylene and polyethylene. However, there is no fundamental understanding of the role intrinsic residual stress plays in the generation of plastic pollutants that threaten the environment and human health. Here, we show that the processing-induced compressive residual stress typically found in polypropylene and polyethylene plastics forces internal nano and microscale segregation of low molecular weight (MW) amorphous polymer droplets onto the plastic's surface. Squeeze flow simulations reveal this stress-driven volumetric flow is consistent with that of a Bingham plastic material, with a temperature-dependent threshold yield stress. We confirm that flow is thermally activated and stress dependent, with a reduced energy barrier at higher compressive stresses. Transfer of surface segregated droplets into water generates amorphous polymer micropollutants (APMPs) that are denatured, with structure and composition different from that of traditional polycrystalline microplastics. Studies with water-containing plastic bottles show that the highly compressed bottle neck and mouth regions are predominantly responsible for the release of APMPs. Our findings reveal a stress-induced mechanism of plastic degradation and underscore the need to modify current plastic processing technologies to reduce residual stress levels and suppress phase separation of low MW APMPs in plastics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
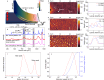


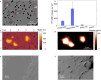
References
-
- Yang, X. et al. Microplastics and chemical leachates from plastic pipes are associated with increased virulence and antimicrobial resistance potential of drinking water microbial communities. J. Hazard. Mater.463, 132900 (2024). - PubMed
-
- Séguéla, R. On the natural draw ratio of semi‐crystalline polymers: review of the mechanical, physical and molecular aspects. Macromol. Mater. Eng.292, 235–244 (2007).
-
- Rogers, J., Dowsett, A., Dennis, P., Lee, J. & Keevil, C. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol.60, 1585–1592 (1994). - PMC - PubMed
-
- Chen, Y. et al. Plastic bottles for chilled carbonated beverages as a source of microplastics and nanoplastics. Water Res.242, 120243 (2023). - PubMed
-
- Nava, V. et al. Plastic debris in lakes and reservoirs. Nature619, 317–322 (2023). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

