Neural representation of cytokines by vagal sensory neurons
- PMID: 40268933
- PMCID: PMC12019601
- DOI: 10.1038/s41467-025-59248-6
Neural representation of cytokines by vagal sensory neurons
Abstract
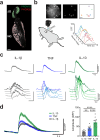
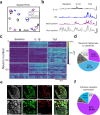
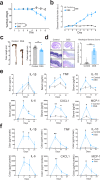
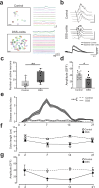
The nervous system coordinates with the immune system to detect and respond to harmful stimuli. Inflammation is a universal response to injury and infection that involves the release of cytokines. While it is known that information about cytokines is transmitted from the body to the brain, how the nervous system encodes specific cytokines in the form of neural activity is not well understood. Using in vivo calcium imaging, we show that vagal sensory neurons within the nodose ganglia exhibit distinct real-time neuronal responses to inflammatory cytokines. Some neurons respond selectively to individual cytokines, while others encode multiple cytokines with distinct activity patterns. In male mice with induced colitis, inflammation increased the baseline activity of these neurons but decreased responsiveness to specific cytokines, reflecting altered neural excitability. Transcriptomic analysis of vagal ganglia from colitis mice revealed downregulation of cytokine signaling pathways, while neuronal activity pathways were upregulated. Thus, nodose ganglia neurons perform real-time encoding of cytokines at the first neural station in a body-brain axis, providing a new framework for studying the dynamic nature of neuroimmune communication.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- R01GM143362/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R35GM118182/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 GM132672/GM/NIGMS NIH HHS/United States
- R01GM132672/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R35 GM118182/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

