A distributed coding logic for thermosensation and inflammatory pain
- PMID: 40269164
- PMCID: PMC12222022
- DOI: 10.1038/s41586-025-08875-6
A distributed coding logic for thermosensation and inflammatory pain
Abstract
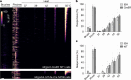
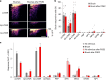
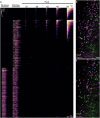
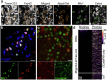
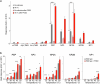
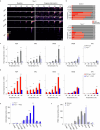
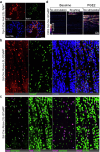
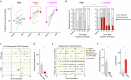
Somatosensory neurons encode detailed information about touch and temperature and are the peripheral drivers of pain1,2. Here by combining functional imaging with multiplexed in situ hybridization3, we determined how heat and mechanical stimuli are encoded across neuronal classes and how inflammation transforms this representation to induce heat hypersensitivity, mechanical allodynia and continuing pain. Our data revealed that trigeminal neurons innervating the cheek exhibited complete segregation of responses to gentle touch and heat. By contrast, heat and noxious mechanical stimuli broadly activated nociceptor classes, including cell types proposed to trigger select percepts and behaviours4-6. Injection of the inflammatory mediator prostaglandin E2 caused long-lasting activity and thermal sensitization in select classes of nociceptors, providing a cellular basis for continuing inflammatory pain and heat hypersensitivity. We showed that the capsaicin receptor TRPV1 (ref. 7) has a central role in heat sensitization but not in spontaneous nociceptor activity. Unexpectedly, the responses to mechanical stimuli were minimally affected by inflammation, suggesting that tactile allodynia results from the continuing firing of nociceptors coincident with touch. Indeed, we have demonstrated that nociceptor activity is both necessary and sufficient for inflammatory tactile allodynia. Together, these findings refine models of sensory coding and discrimination at the cellular and molecular levels, demonstrate that touch and temperature are broadly but differentially encoded across transcriptomically distinct populations of sensory cells and provide insight into how cellular-level responses are reshaped by inflammation to trigger diverse aspects of pain.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

