CMV serostatus is associated with improved survival and delayed toxicity onset following anti-PD-1 checkpoint blockade
- PMID: 40269332
- PMCID: PMC12283384
- DOI: 10.1038/s41591-025-03647-1
CMV serostatus is associated with improved survival and delayed toxicity onset following anti-PD-1 checkpoint blockade
Abstract
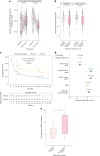
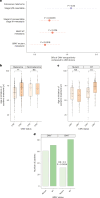
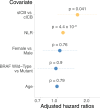
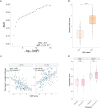
Cytomegalovirus (CMV) is a globally endemic latent herpes virus that profoundly impacts T cell immunity. We investigated the oncological consequences of CMV infection across 341 prospectively recruited patients receiving immune checkpoint blockade (ICB) for melanoma. CMV+ patients with metastatic melanoma (MM) have higher lymphocyte counts, reduced neutrophil to lymphocyte ratio and divergent CD8+ T cell gene expression. Combination anti-CTLA-4/anti-PD-1 ICB, but not single-agent anti-PD-1 ICB, induces cytotoxicity and CMV-associated gene expression in CD8+ T cells from CMV- patients. Correspondingly, overall survival was independent of CMV serostatus in combination anti-CTLA-4/anti-PD-1 ICB recipients (CMV+ hazard ratio for death: 1.02, P = 0.92), whereas CMV+ single-agent anti-PD-1 ICB recipients had improved overall survival (CMV+ hazard ratio for death: 0.37, P < 0.01), a finding also seen in CMV+ adjuvant single-agent anti-PD-1 ICB recipients (CMV+ hazard ratio for recurrence: 0.19, P = 0.03). We identify TBX21, encoding T-bet, as a transcriptional driver of CMV-associated CD8+ T cell gene expression, finding that TBX21 expression is predictive of overall survival (hazard ratio: 0.62, P = 0.026). CMV+ patients unexpectedly show reduced cumulative incidence of grade 3+ immune-related adverse events at 6 months (0.30 versus 0.52, P = 2.2 × 10-5), with lower incidence of colitis (P = 7.8 × 10-4) and pneumonitis (P = 0.028), an effect replicated in non-melanoma ICB recipients (n = 58, P = 0.044). Finally, we find reduced CMV seropositivity rates in patients with MM compared with UK Biobank controls (odds ratio: 0.52, P = 1.8 × 10-4), indicating CMV seropositivity may protect against MM. Specifically, patients with BRAF-mutated MM are less likely to be CMV+ (odds ratio = 2.2, P = 0.0054), while CMV- patients present 9 yr earlier with BRAF wild-type MM (P = 1.3 × 10-4). This work reveals an interaction between CMV infection, MM development according to BRAF status and response to ICB, while demonstrating CMV infection is protective against severe ICB immune-related adverse events, highlighting the potential importance of previous infection history and chronic immune activation in MM development and immunotherapy outcomes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: In the last 3 years, B.P.F. has performed consultancy for NICE Consultancy, Roche, Pathios, UCB and TCypher and has been given speaker fees by GSK, UCB and BMS, all outside the submitted work. M.R.M. reports grants from Roche, grants from Astrazeneca, grants from GSK, expenses from Novartis, grants and expenses from Immunocore, expenses from BMS, expenses from Pfizer, expenses from Merck/MSD, expenses from Regeneron, expenses from BiolineRx, expenses from Replimune and grants from GRAIL, all outside the submitted work. The other authors declare no competing interests.
Figures







References
-
- Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature557, 575–579 (2018). - PubMed
-
- Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med.25, 89–94 (2019). - PubMed
-
- Wu, T. D. et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature579, 274–278 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

