Cognitive behavioral therapy skills via a smartphone app for subthreshold depression among adults in the community: the RESiLIENT randomized controlled trial
- PMID: 40269333
- PMCID: PMC12176654
- DOI: 10.1038/s41591-025-03639-1
Cognitive behavioral therapy skills via a smartphone app for subthreshold depression among adults in the community: the RESiLIENT randomized controlled trial
Abstract
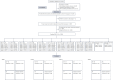
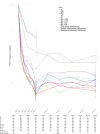
Subthreshold depression, defined as a depressive status falling short of the diagnostic threshold for major depression, is common, disabling and constitutes a risk factor for future depressive episodes. Cognitive behavioral therapies (CBT) have been shown to be effective but are usually provided as packages of various skills. Little research has been done to investigate whether all their components are beneficial and contributory to mental health promotion. We addressed this issue by developing a smartphone CBT app that implements five representative CBT skills (behavioral activation, cognitive restructuring, problem solving, assertion training and behavior therapy for insomnia), and conducting a master randomized study that included four 2 × 2 factorial trials to enable precise estimation of skill-specific efficacies. Between September 2022 and February 2024, we recruited 3,936 adult participants with subthreshold depression. Among those randomized, the follow-up rate was 97% at week 6 and adherence to the app was 84%. The study showed that all included CBT skills and their combinations differentially beat all three control conditions of delayed treatment, health information or self-check, with effect sizes ranging between -0.67 (95% confidence interval: -0.81 to -0.53) and -0.16 (-0.30 to -0.02) for changes in depressive symptom severity from baseline to week 6, as measured with the Patient Health Questionnaire-9 scores. Knowledge of the active ingredients of CBT can better inform the design of more effective and scalable psychotherapies in the future. (UMIN Clinical Trials Registry UMIN000047124 ).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.A.F. reports personal fees from Boehringer Ingelheim, Daiichi Sankyo, DT Axis, Micron, Shionogi, SONY and UpToDate, and a grant from DT Axis and Shionogi, outside the submitted work. In addition, T.A.F. has a patent (7448125) and a pending patent (2022-082495), and has licensed intellectual properties for Kokoro-app to DT Axis. A.T. reports personal fees from Eisai and Shionogi outside the submitted work. M.S. is employed in the Department of Neurodevelopmental Disorders, Nagoya City University Graduate School of Medical Sciences, which is an endowment department supported by the City of Nagoya, and has received a personal fee from SONY outside the submitted work. M.H. has a patent (7448125) and has licensed intellectual properties for Kokoro-app to Mitsubishi Tanabe. T.A. has received lecture fees from AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Kowa, Kyowa Kirin, Lundbeck, MSD, Meiji-Seika Pharma, Merck, Nipro, Otsuka, Pfizer, Shionogi, Sumitomo pharma, Takeda, Tsumura, UCB and Viatris, and a grant from Shinogi and royalties from Igaku-Shoin, outside the submitted work. T.A. serves as the representative director of the General Incorporated Association NCU CRESS and receives compensation as an advisor to Snom Inc. T.A. has pending patents (2020-135195 and 2024-516288) (Institute) and patents (7313617) (Institute). N. Kawakami is employed by the Junpukai Foundation and the Department of Digital Mental Health, The University of Tokyo, an endowment department that is supported by an unrestricted grant from 15 enterprises ( https://dmh.m.u-tokyo.ac.jp/en ). T.N. reports grants or contracts with I&H, Cocokarafine, Konica Minolta and NTT DATA, consulting fees from Ohtsuka, Takeda, Johnson & Johnson, AstraZeneca and Nippon Zoki, payment or honoraria for lectures, manuscript writing or educational events from Pfizer, MSD, Chugai, Takeda, Janssen, Boehringer Ingelheim, Eli Lilly, Maruho, Mitsubishi Tanabe, Novartis, Allergan, Novo Nordisk, Toa Eiyo, AbbVie, Ono, GSK, Alexion, Cannon Medical Systems, Kowa, Araya, Merck and Amicus, stock options in BonBon Inc., and a donation from Cancerscan and JMDC, all outside the submitted work. S.F. received personal fees from Kyowa Kirin, Boehringer Ingelheim, Toray Medical, Health and Global Policy Institute, White Healthcare and Health Insurance for Start-up Companies, and research grants from the Japan Health Insurance Association, National Health Insurance Association for Civil Engineering and Construction, Osaka Prefecture and Cancerscan, outside this work. R.C.K. was a consultant for Cambridge Health Alliance, Canandaigua VA Medical Center, Child Mind Institute, Holmusk, Massachusetts General Hospital, Partners Healthcare, Inc., RallyPoint Networks, Inc., Sage Therapeutics and University of North Carolina. He has stock options in Cerebral Inc., Mirah, PYM (Prepare Your Mind), Roga Sciences and Verisense Health. The other authors declare no competing interests.
Figures

References
-
- Sigstrom, R., Waern, M., Gudmundsson, P., Skoog, I. & Ostling, S. Depressive spectrum states in a population-based cohort of 70-year olds followed over 9 years. Int. J. Geriatr. Psychiatry33, 1028–1037 (2018). - PubMed
-
- Karsten, J., Penninx, B. W., Verboom, C. E., Nolen, W. A. & Hartman, C. A. Course and risk factors of functional impairment in subthreshold depression and anxiety. Depress. Anxiety30, 386–394 (2013). - PubMed
-
- Backenstrass, M. et al. A comparative study of nonspecific depressive symptoms and minor depression regarding functional impairment and associated characteristics in primary care. Compr. Psychiatry47, 35–41 (2006). - PubMed

