Real-world antibiotic utilization during pregnancy in Italy: a multiregional retrospective population-based study
- PMID: 40269796
- PMCID: PMC12020250
- DOI: 10.1186/s12884-025-07605-0
Real-world antibiotic utilization during pregnancy in Italy: a multiregional retrospective population-based study
Abstract
Background: Exposure to antibiotics during pregnancy is frequent, despite the limited evidence derived from clinical trials. Drug utilization studies could improve knowledge on utilization of these medications during this critical period. In this context, the present study aimed to describe antibiotic exposure during pregnancy in Italy at both national and regional levels.
Methods: This retrospective population-based study involved a cohort of women who gave birth from 2016 to 2018 and were residents of one of the following Italian regions: Lombardy, Veneto, Emilia-Romagna, Tuscany, Umbria, Lazio, Apulia or Sardinia. A series of sociodemographic and clinical characteristics were retrieved from regional healthcare databases. The prevalence of the use of antibiotics was estimated in nine trimesters, which were divided into three different periods: pre- pregnancy (-III, -II, -I) during pregnancy (I, II, III) and post-pregnancy (+ I, + II, + III). Analyses were stratified by region and by prenatal invasive diagnostic performed.
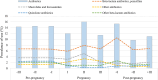
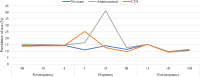
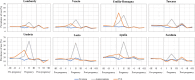
Results: A total of 449,012 women were included in the study, of whom more than 37% were aged ≥ 35 years at birth. The overall prevalence rates of antibiotic use in the study cohort were 33.9% pre-pregnancy (per trimester: -III = 14.3%, -II = 14.5%, -I = 14.5%), 31.8% during pregnancy (per trimester: I = 12.0%, II = 16.0%, III = 11.4%) and 29.3% post-pregnancy (per trimester: + I = 15.3%, + II = 9.7%; + III = 11.0%). The regions with the lowest usage pre-, during and post-pregnancy were Lombardy (29.7%, 26.1%, 28.0%) and Veneto (28.8%, 26.4%, 25.5%), whereas Apulia reached the highest values (45.6%, 41.6%, 38.3%). The highest peaks during pregnancy were reached by Umbria (25.8%), Latium (24.1%) and Apulia (21.4%). Women who underwent chorionic villus sampling and those who underwent amniocentesis registered a peak during trimester I (25%) and trimester II (41%), respectively. These peaks were in line with the timing of the invasive prenatal diagnostic procedures.
Conclusions: The use of antibiotics during pregnancy in Italy was in line with other European countries, reflecting national and international guidelines. However, a certain level of misuse of specific antibiotics and different utilization rates across the regions were observed. Continuous monitoring of long- and short-term outcomes associated with exposure to antibiotics during pregnancy may contribute to reducing excessive utilization and improving the diffusion of more appropriate procedures and practices.
Keywords: Antibiotics; Misuse; Monitoring; Pharmacoutilization; Pregnancy; Prenatal diagnostic procedures; Prevalence of use; Real-world practice; Variability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This was a retrospective, descriptive cohort study based on the linkage among regional healthcare databases. The eight regions involved provided datasets containing only fully anonymized aggregated data, which were strictly necessary for the pre-planned analyses. According to the Italian Legislative Decree No. 196/2003 (Personal Data Protection Code) and EU Regulation No. 45/2001 on the protection of personal data, ethical approval and informed consent were not required for this study. Although the study was conducted prior to the enforcement of the General Data Protection Regulation (GDPR) in May 2018, it is compliant with its principles as a standard for data protection. The study adhered to the principles of the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

