Cellular Activity Modulation Mediated by Near Infrared-Irradiated Polydopamine Nanoparticles: In Vitro and Ex Vivo Investigation
- PMID: 40270300
- PMCID: PMC12060647
- DOI: 10.1021/acsnano.5c04181
Cellular Activity Modulation Mediated by Near Infrared-Irradiated Polydopamine Nanoparticles: In Vitro and Ex Vivo Investigation
Abstract
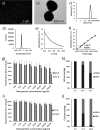
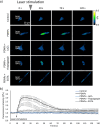
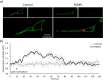
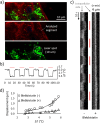
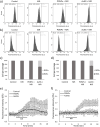
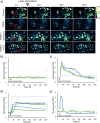
The precise control of cell activity is crucial for understanding and potentially treating many disorders. Focusing on neurons and myotubes, recent advancements in nanotechnology have introduced photoresponsive nanoparticles as an alternative tool for modulating cell function with high spatial and temporal resolution. This approach offers a noninvasive alternative to traditional stimulation techniques, reducing potential tissue damage and improving the specificity of cell activation. Here, we introduce an approach envisioning fully organic polydopamine nanoparticles (PDNPs) to remotely modulate the activity of differentiated SH-SY5Y cells and differentiated C2C12 cells, via near-infrared (NIR) laser stimulation. Confocal microscopy imaging revealed the possibility of thermally activating individual neuron-like cells, eliciting a significant cellular response characterized by the generation of calcium transients and the subsequent release of the neurotransmitter acetylcholine. Similarly, we demonstrated the possibility of precisely triggering the muscle contraction of single myotubes. Additionally, we investigated the antioxidant properties of PDNPs, demonstrating their capacity to prevent an increase in oxidative stress levels related to an increase in intracellular temperature. Moreover, proteomic analysis revealed that a PDNP treatment could positively affect neuronal plasticity and nervous system maturation, besides promoting muscle growth and preserving its functional integrity, underscoring its potential to support both neural and musculoskeletal development. Eventually, the effect of the NIR laser irradiation in the presence of PDNPs in neuron-like cells was successfully evaluated ex vivo on brains of Drosophila melanogaster, genetically modified to express the fluorescent calcium indicator jGCaMP7c.
Keywords: Drosophila melanogaster; acetylcholine release; cell activity modulation; photothermal stimulation; polydopamine nanoparticles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Polydopamine Nanoparticles as an Organic and Biodegradable Multitasking Tool for Neuroprotection and Remote Neuronal Stimulation.ACS Appl Mater Interfaces. 2020 Aug 12;12(32):35782-35798. doi: 10.1021/acsami.0c05497. Epub 2020 Aug 3. ACS Appl Mater Interfaces. 2020. PMID: 32693584 Free PMC article.
-
Detailed Profiling of Protein Corona Formed by Polydopamine Nanoparticles in Human Plasma.ACS Appl Mater Interfaces. 2025 Feb 19;17(7):10485-10498. doi: 10.1021/acsami.4c21207. Epub 2025 Feb 5. ACS Appl Mater Interfaces. 2025. PMID: 39909726
-
Construction of Surface-Modified Polydopamine Nanoparticles for Sequential Drug Release and Combined Chemo-Photothermal Cancer Therapy.Mol Pharm. 2021 Mar 1;18(3):1327-1343. doi: 10.1021/acs.molpharmaceut.0c01164. Epub 2021 Feb 2. Mol Pharm. 2021. PMID: 33530691
-
Self-Assembled Dual-Targeted Epirubicin-Hybrid Polydopamine Nanoparticles for Combined Chemo-Photothermal Therapy of Triple-Negative Breast Cancer.Int J Nanomedicine. 2020 Sep 11;15:6791-6811. doi: 10.2147/IJN.S260477. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32982234 Free PMC article.
-
Surface Engineering of Metal-Organic Framework as pH-/NIR-Responsive Nanocarrier for Imaging-Guided Chemo-Photothermal Therapy.Int J Nanomedicine. 2020 May 7;15:3235-3250. doi: 10.2147/IJN.S239910. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32440121 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

